Adaptive behavior of bacterial mechanosensitive channels is coupled to membrane mechanics
- PMID: 20513760
- PMCID: PMC2888061
- DOI: 10.1085/jgp.200910371
Adaptive behavior of bacterial mechanosensitive channels is coupled to membrane mechanics
Abstract
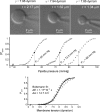
Mechanosensitive channel of small conductance (MscS), a tension-driven osmolyte release valve residing in the inner membrane of Escherichia coli, exhibits a complex adaptive behavior, whereas its functional counterpart, mechanosensitive channel of large conductance (MscL), was generally considered nonadaptive. In this study, we show that both channels exhibit similar adaptation in excised patches, a process that is completely separable from inactivation prominent only in MscS. When a membrane patch is held under constant pressure, adaptation of both channels is manifested as a reversible current decline. Their dose-response curves recorded with 1-10-s ramps of pressure are shifted toward higher tension relative to the curves measured with series of pulses, indicating decreased tension sensitivity. Prolonged exposure of excised patches to subthreshold tensions further shifts activation curves for both MscS and MscL toward higher tension with similar magnitude and time course. Whole spheroplast MscS recordings performed with simultaneous imaging reveal activation curves with a midpoint tension of 7.8 mN/m and the slope corresponding to approximately 15-nm(2) in-plane expansion. Inactivation was retained in whole spheroplast mode, but no adaptation was observed. Similarly, whole spheroplast recordings of MscL (V23T mutant) indicated no adaptation, which was present in excised patches. MscS activities tried in spheroplast-attached mode showed no adaptation when the spheroplasts were intact, but permeabilized spheroplasts showed delayed adaptation, suggesting that the presence of membrane breaks or edges causes adaptation. We interpret this in the framework of the mechanics of the bilayer couple linking adaptation of channels in excised patches to the relaxation of the inner leaflet that is not in contact with the glass pipette. Relaxation of one leaflet results in asymmetric redistribution of tension in the bilayer that is less favorable for channel opening.
Figures





Similar articles
-
Effects of GsMTx4 on bacterial mechanosensitive channels in inside-out patches from giant spheroplasts.Biophys J. 2010 Nov 3;99(9):2870-8. doi: 10.1016/j.bpj.2010.09.022. Biophys J. 2010. PMID: 21044584 Free PMC article.
-
The mechanoelectrical response of the cytoplasmic membrane of Vibrio cholerae.J Gen Physiol. 2013 Jul;142(1):75-85. doi: 10.1085/jgp.201310985. J Gen Physiol. 2013. PMID: 23797422 Free PMC article.
-
Characterizing the mechanosensitive response of Paraburkholderia graminis membranes.Biochim Biophys Acta Biomembr. 2020 Apr 1;1862(4):183176. doi: 10.1016/j.bbamem.2020.183176. Epub 2020 Jan 7. Biochim Biophys Acta Biomembr. 2020. PMID: 31923411
-
State-stabilizing Interactions in Bacterial Mechanosensitive Channel Gating and Adaptation.J Biol Chem. 2009 Jul 17;284(29):19153-7. doi: 10.1074/jbc.R109.009357. Epub 2009 Apr 21. J Biol Chem. 2009. PMID: 19383606 Free PMC article. Review.
-
Mechanosensitive channels: what can they do and how do they do it?Structure. 2011 Oct 12;19(10):1356-69. doi: 10.1016/j.str.2011.09.005. Structure. 2011. PMID: 22000509 Free PMC article. Review.
Cited by
-
The pathway and spatial scale for MscS inactivation.J Gen Physiol. 2011 Jul;138(1):49-57. doi: 10.1085/jgp.201110606. Epub 2011 Jun 13. J Gen Physiol. 2011. PMID: 21670207 Free PMC article.
-
Spatiotemporal relationships defining the adaptive gating of the bacterial mechanosensitive channel MscS.Eur Biophys J. 2018 Sep;47(6):663-677. doi: 10.1007/s00249-018-1303-5. Epub 2018 Apr 23. Eur Biophys J. 2018. PMID: 29687344
-
Electrophysiological characterization of the mechanosensitive channel MscCG in Corynebacterium glutamicum.Biophys J. 2013 Sep 17;105(6):1366-75. doi: 10.1016/j.bpj.2013.06.054. Biophys J. 2013. PMID: 24047987 Free PMC article.
-
MscS-like mechanosensitive channels in plants and microbes.Biochemistry. 2013 Aug 27;52(34):5708-22. doi: 10.1021/bi400804z. Epub 2013 Aug 15. Biochemistry. 2013. PMID: 23947546 Free PMC article. Review.
-
Integrating the biophysical and molecular mechanisms of auditory hair cell mechanotransduction.Nat Commun. 2011 Nov 1;2:523. doi: 10.1038/ncomms1533. Nat Commun. 2011. PMID: 22045002 Free PMC article. Review.
References
-
- Baoukina S.V., Mukhin S.I. 2004. Bilayer membrane in confined geometry: interlayer slide and entropic repulsion. J. Exp. Theor. Phys. 99:875–888 10.1134/1.1826180 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

