Proton block of the CLC-5 Cl-/H+ exchanger
- PMID: 20513761
- PMCID: PMC2888053
- DOI: 10.1085/jgp.201010428
Proton block of the CLC-5 Cl-/H+ exchanger
Abstract
CLC-5 is a H(+)/Cl(-) exchanger that is expressed primarily in endosomes but can traffic to the plasma membrane in overexpression systems. Mutations altering the expression or function of CLC-5 lead to Dent's disease. Currents mediated by this transporter show extreme outward rectification and are inhibited by acidic extracellular pH. The mechanistic origins of both phenomena are currently not well understood. It has been proposed that rectification arises from the voltage dependence of a H(+) transport step, and that inhibition of CLC-5 currents by low extracellular pH is a result of a reduction in the driving force for exchange caused by a pH gradient. We show here that the pH dependence of CLC-5 currents arises from H(+) binding to a single site located halfway through the transmembrane electric field and driving the transport cycle in a less permissive direction, rather than a reduction in the driving force. We propose that protons bind to the extracellular gating glutamate E211 in CLC-5. It has been shown that CLC-5 becomes severely uncoupled when SCN(-) is the main charge carrier: H(+) transport is drastically reduced while the rate of anion movement is increased. We found that in these conditions, rectification and pH dependence are unaltered. This implies that H(+) translocation is not the main cause of rectification. We propose a simple transport cycle model that qualitatively accounts for these findings.
Figures
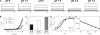



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

