Regulation of basal cellular physiology by the homeostatic unfolded protein response
- PMID: 20513765
- PMCID: PMC2878945
- DOI: 10.1083/jcb.201003138
Regulation of basal cellular physiology by the homeostatic unfolded protein response
Abstract
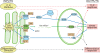
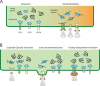
The extensive membrane network of the endoplasmic reticulum (ER) is physically juxtaposed to and functionally entwined with essentially all other cellular compartments. Therefore, the ER must sense diverse and constantly changing physiological inputs so it can adjust its numerous functions to maintain cellular homeostasis. A growing body of new work suggests that the unfolded protein response (UPR), traditionally charged with signaling protein misfolding stress from the ER, has been co-opted for the maintenance of basal cellular homeostasis. Thus, the UPR can be activated, and its output modulated, by signals far outside the realm of protein misfolding. These findings are revealing that the UPR causally contributes to disease not just by its role in protein folding but also through its broad influence on cellular physiology.
Figures

References
-
- Back S.H., Scheuner D., Han J., Song B., Ribick M., Wang J., Gildersleeve R.D., Pennathur S., Kaufman R.J. 2009. Translation attenuation through eIF2alpha phosphorylation prevents oxidative stress and maintains the differentiated state in beta cells. Cell Metab. 10:13–26 10.1016/j.cmet.2009.06.002 - DOI - PMC - PubMed
-
- Baltzis D., Qu L.K., Papadopoulou S., Blais J.D., Bell J.C., Sonenberg N., Koromilas A.E. 2004. Resistance to vesicular stomatitis virus infection requires a functional cross talk between the eukaryotic translation initiation factor 2alpha kinases PERK and PKR. J. Virol. 78:12747–12761 10.1128/JVI.78.23.12747-12761.2004 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

