Multiple mechanisms collectively regulate clathrin-mediated endocytosis of the epidermal growth factor receptor
- PMID: 20513767
- PMCID: PMC2878939
- DOI: 10.1083/jcb.201001008
Multiple mechanisms collectively regulate clathrin-mediated endocytosis of the epidermal growth factor receptor
Abstract
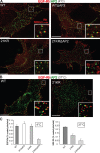
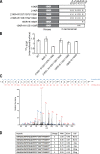
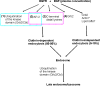
Endocytosis of the epidermal growth factor receptor (EGFR) is important for the regulation of EGFR signaling. However, EGFR endocytosis mechanisms are poorly understood, which precludes development of approaches to specifically inhibit EGFR endocytosis and analyze its impact on signaling. Using a combination of receptor mutagenesis and RNA interference, we demonstrate that clathrin-dependent internalization of activated EGFR is regulated by four mechanisms, which function in a redundant and cooperative fashion. These mechanisms involve ubiquitination of the receptor kinase domain, the clathrin adaptor complex AP-2, the Grb2 adaptor protein, and three C-terminal lysine residues (K1155, K1158, and K1164), which are acetylated, a novel posttranslational modification for the EGFR. Based on these findings, the first internalization-defective EGFR mutant with functional kinase and normal tyrosine phosphorylation was generated. Analysis of the signaling kinetics of this mutant revealed that EGFR internalization is required for the sustained activation of protein kinase B/AKT but not for the activation of mitogen-activated protein kinase.
Figures



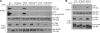


References
-
- Chang C.-P., Lazar C.S., Walsh B.J., Komuro M., Collawn J.F., Kuhn L.A., Tainer J.A., Trowbridge I.S., Farquhar M.G., Rosenfeld M.G., et al. 1993. Ligand-induced internalization of the epidermal growth factor receptor is mediated by multiple endocytic codes analogous to the tyrosine motif found in constitutively internalized receptors. J. Biol. Chem. 268:19312–19320 - PubMed
-
- Chen W.S., Lazar C.S., Lund K.A., Welsh J.B., Chang C.P., Walton G.M., Der C.J., Wiley H.S., Gill G.N., Rosenfeld M.G. 1989. Functional independence of the epidermal growth factor receptor from a domain required for ligand-induced internalization and calcium regulation. Cell. 59:33–43 10.1016/0092-8674(89)90867-2 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

