Isocitrate dehydrogenase 1 and 2 mutations in cancer: alterations at a crossroads of cellular metabolism
- PMID: 20513808
- PMCID: PMC2897878
- DOI: 10.1093/jnci/djq187
Isocitrate dehydrogenase 1 and 2 mutations in cancer: alterations at a crossroads of cellular metabolism
Abstract
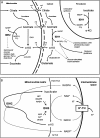
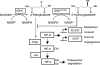
Dysregulation of metabolism is a common phenomenon in cancer cells. The NADP(+)-dependent isocitrate dehydrogenases 1 and 2 (IDH1 and IDH2) function at a crossroads of cellular metabolism in lipid synthesis, cellular defense against oxidative stress, oxidative respiration, and oxygen-sensing signal transduction. We review the normal functions of the encoded enzymes, frequent mutations of IDH1 and IDH2 recently found in human cancers, and possible roles for the mutated enzymes in human disease. IDH1 and IDH2 mutations occur frequently in some types of World Health Organization grades 2-4 gliomas and in acute myeloid leukemias with normal karyotype. IDH1 and IDH2 mutations are remarkably specific to codons that encode conserved functionally important arginines in the active site of each enzyme. To date, all IDH1 mutations have been identified at the Arg132 codon. Mutations in IDH2 have been identified at the Arg140 codon, as well as at Arg172, which is aligned with IDH1 Arg132. IDH1 and IDH2 mutations are usually heterozygous in cancer, and they appear to confer a neomorphic enzyme activity for the enzymes to catalyze the production of D-2-hydroxyglutarate. Study of alterations in these metabolic enzymes may provide insights into the metabolism of cancer cells and uncover novel avenues for development of anticancer therapeutics.
Figures
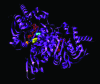

References
-
- Gabriel JL, Zervos PR, Plaut GW. Activity of purified NAD-specific isocitrate dehydrogenase at modulator and substrate concentrations approximating conditions in mitochondria. Metabolism. 1986;35(7):661–667. - PubMed
-
- Jennings GT, Sechi S, Stevenson PM, Tuckey RC, Parmelee D, McAlister-Henn L. Cytosolic NADP(+)-dependent isocitrate dehydrogenase. Isolation of rat cDNA and study of tissue-specific and developmental expression of mRNA. J Biol Chem. 1994;269(37):23128–23134. - PubMed
-
- Nekrutenko A, Hillis DM, Patton JC, Bradley RD, Baker RJ. Cytosolic isocitrate dehydrogenase in humans, mice, and voles and phylogenetic analysis of the enzyme family. Mol Biol Evol. 1998;15(12):1674–1684. - PubMed
-
- Geisbrecht BV, Gould SJ. The human PICD gene encodes a cytoplasmic and peroxisomal NADP(+)-dependent isocitrate dehydrogenase. J Biol Chem. 1999;274(43):30527–30533. - PubMed
-
- Henke B, Girzalsky W, Berteaux-Lecellier V, Erdmann R. IDP3 encodes a peroxisomal NADP-dependent isocitrate dehydrogenase required for the beta-oxidation of unsaturated fatty acids. J Biol Chem. 1998;273(6):3702–3711. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

