Transgenic expression of 15-lipoxygenase 2 (15-LOX2) in mouse prostate leads to hyperplasia and cell senescence
- PMID: 20514017
- PMCID: PMC3042242
- DOI: 10.1038/onc.2010.197
Transgenic expression of 15-lipoxygenase 2 (15-LOX2) in mouse prostate leads to hyperplasia and cell senescence
Abstract
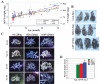
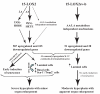
15-Lipoxygenase 2 (15-LOX2), a lipid-peroxidizing enzyme, is mainly expressed in the luminal compartment of the normal human prostate, and is often decreased or lost in prostate cancer. Previous studies from our lab implicate 15-LOX2 as a functional tumor suppressor. To better understand the biological role of 15-LOX2 in vivo, we generated prostate-specific 15-LOX2 transgenic mice using the ARR2PB promoter. Unexpectedly, transgenic expression of 15-LOX2 or 15-LOX2sv-b, a splice variant that lacks arachidonic acid-metabolizing activity, resulted in age-dependent prostatic hyperplasia and enlargement of the prostate. Prostatic hyperplasia induced by both 15-LOX2 and 15-LOX2sv-b was associated with an increase in luminal and Ki-67(+) cells; however, 15-LOX2-transgenic prostates also showed a prominent increase in basal cells. Microarray analysis revealed distinct gene expression profiles that could help explain the prostate phenotypes. Strikingly, 15-LOX2, but not 15-LOX2sv-b, transgenic prostate showed upregulation of several well-known stem or progenitor cell molecules including Sca-1, Trop2, p63, Nkx3.1 and Psca. Prostatic hyperplasia caused by both 15-LOX2 and 15-LOX2sv-b did not progress to prostatic intraprostate neoplasia or carcinoma and, mechanistically, prostate lobes (especially those of 15-LOX2 mice) showed a dramatic increase in senescent cells as revealed by increased SA-betagal, p27(Kip1) and heterochromatin protein 1gamma staining. Collectively, our results suggest that 15-LOX2 expression in mouse prostate leads to hyperplasia and also induces cell senescence, which may, in turn, function as a barrier to tumor development.
Figures





References
-
- Abate-Shen C, Shen MM. Molecular genetics of prostate cancer. Genes Dev. 2000;14:2410–34. - PubMed
-
- Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11:155–68. - PubMed
-
- Bhatia B, Maldonado CJ, Tang S, Chandra D, Klein RD, Chopra D, et al. Subcellular localization and tumor-suppressive functions of 15-lipoxygenase 2 (15-LOX2) and its splice variants. J Biol Chem. 2003;278:25091–100. - PubMed
-
- Bhatia B, Tang S, Yang P, Doll A, Aumueller G, Newman RA, et al. Cell-autonomous induction of functional tumor suppressor 15-lipoxygenase 2 (15-LOX2) contributes to replicative senescence of human prostate progenitor cells. Oncogene. 2005;24:3583–95. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

