Metabolism and proliferation share common regulatory pathways in cancer cells
- PMID: 20514019
- PMCID: PMC3004916
- DOI: 10.1038/onc.2010.182
Metabolism and proliferation share common regulatory pathways in cancer cells
Abstract
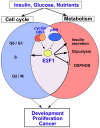
Cancer development involves major alterations in cells' metabolism. Enhanced glycolysis and de novo fatty acids synthesis are indeed characteristic features of cancer. Cell proliferation and metabolism are tightly linked cellular processes. Others and we have previously shown a close relationship between metabolic responses and proliferative stimuli. In addition to trigger proliferative and survival signaling pathways, most oncoproteins also trigger metabolic changes to transform the cell. We present herein the view that participation of cell-cycle regulators and oncogenic proteins to cancer development extend beyond the control of cell proliferation, and discuss how these new functions may be implicated in metabolic alterations concomitant to the pathogenesis of human cancers.
Figures


References
-
- Abella A, Dubus P, Malumbres M, Rane SG, Kiyokawa H, Sicard A, et al. Cdk4 promotes adipogenesis through PPARgamma activation. Cell Metab. 2005;2:239–249. - PubMed
-
- Barthel A, Okino ST, Liao J, Nakatani K, Li J, Whitlock JP, Jr, et al. Regulation of GLUT1 gene transcription by the serine/threonine kinase Akt1. J Biol Chem. 1999;274:20281–20286. - PubMed
-
- Bauer DE, Hatzivassiliou G, Zhao F, Andreadis C, Thompson CB. ATP citrate lyase is an important component of cell growth and transformation. Oncogene. 2005;24:6314–6322. - PubMed
-
- Berwick DC, Hers I, Heesom KJ, Moule SK, Tavare JM. The identification of ATP-citrate lyase as a protein kinase B (Akt) substrate in primary adipocytes. J Biol Chem. 2002;277:33895–33900. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

