Cellular bioenergetics as a target for obesity therapy
- PMID: 20514071
- PMCID: PMC2880836
- DOI: 10.1038/nrd3138
Cellular bioenergetics as a target for obesity therapy
Abstract
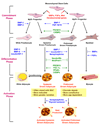
Obesity develops when energy intake exceeds energy expenditure. Although most current obesity therapies are focused on reducing calorific intake, recent data suggest that increasing cellular energy expenditure (bioenergetics) may be an attractive alternative approach. This is especially true for adaptive thermogenesis - the physiological process whereby energy is dissipated in mitochondria of brown fat and skeletal muscle in the form of heat in response to external stimuli. There have been significant recent advances in identifying the factors that control the development and function of these tissues, and in techniques to measure brown fat in human adults. In this article, we integrate these developments in relation to the classical understandings of cellular bioenergetics to explore the potential for developing novel anti-obesity therapies that target cellular energy expenditure.
Figures



References
-
- Haslam DW, James WP. Obesity. Lancet. 2005;366:1197–1209. - PubMed
-
- Rolfe DF, Brown GC. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol. Rev. 1997;77:731–758. - PubMed
-
- Lowell BB, Spiegelman BM. Towards a molecular understanding of adaptive thermogenesis. Nature. 2000;404:652–660. - PubMed
-
- Wing RR, Phelan S. Long-term weight loss maintenance. Am J Clin Nutr. 2005;82 222S–225S. - PubMed
-
- Kaplan LM. Pharmacological therapies for obesity. Gastroenterol. Clin North Am. 2005;34:91–104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK081604/DK/NIDDK NIH HHS/United States
- DK046200/DK/NIDDK NIH HHS/United States
- DK082659/DK/NIDDK NIH HHS/United States
- R01 DK077097/DK/NIDDK NIH HHS/United States
- RR025758/RR/NCRR NIH HHS/United States
- K23 DK081604/DK/NIDDK NIH HHS/United States
- UL1 RR025758/RR/NCRR NIH HHS/United States
- R01 DK082659/DK/NIDDK NIH HHS/United States
- KL2 RR025757/RR/NCRR NIH HHS/United States
- P30 DK046200/DK/NIDDK NIH HHS/United States
- P30 DK036836/DK/NIDDK NIH HHS/United States
- DK077097/DK/NIDDK NIH HHS/United States
- RR025757/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

