Kicked by Mos and tuned by MPF-the initiation of the MAPK cascade in Xenopus oocytes
- PMID: 20514133
- PMCID: PMC2839814
- DOI: 10.2976/1.3265771
Kicked by Mos and tuned by MPF-the initiation of the MAPK cascade in Xenopus oocytes
Abstract
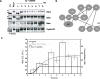
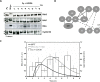
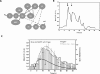
The mitogen-activated protein kinase (MAPK) cascade is a paradigmatic signaling cascade, which plays a crucial role in many aspects of cellular events. The main initiator of the cascade in Xenopus oocytes is the oncoprotein Mos. After activation of the cascade, Mos activity is stabilized by MAPK via a feedback loop. Mos concentration levels are, however, not controlled by MAPK alone. In this paper we show, by imposing either a sustained or a peaked activity of M-phase promoting factor (MPF) (Cdc2-cyclin B), how the latter regulates the dynamics of Mos. Our experiments are supported by a detailed kinetic model for the Mos-MPF-MAPK network, which takes into account the three different phosphorylation states of Mos and, as a consequence, allows us to determine the time evolution of Mos under control of MPF. Our work opens a path toward a more complete and biologically realistic quantitative understanding of the dynamic interdependence of Mos and MPF in Xenopus oocytes.
Figures


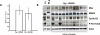
Similar articles
-
Interplay between Cdc2 kinase and the c-Mos/MAPK pathway between metaphase I and metaphase II in Xenopus oocytes.Dev Biol. 2001 Mar 1;231(1):279-88. doi: 10.1006/dbio.2000.0142. Dev Biol. 2001. PMID: 11180968
-
The mitogen-activated protein kinase signaling pathway stimulates mos mRNA cytoplasmic polyadenylation during Xenopus oocyte maturation.Mol Cell Biol. 1999 Mar;19(3):1990-9. doi: 10.1128/MCB.19.3.1990. Mol Cell Biol. 1999. PMID: 10022886 Free PMC article.
-
Initiation of Xenopus oocyte maturation by activation of the mitogen-activated protein kinase cascade.J Biol Chem. 1995 Oct 27;270(43):25898-904. doi: 10.1074/jbc.270.43.25898. J Biol Chem. 1995. PMID: 7592777
-
c-Mos and cyclin B/cdc2 connections during Xenopus oocyte maturation.Biol Cell. 2001 Sep;93(1-2):15-25. doi: 10.1016/s0248-4900(01)01128-5. Biol Cell. 2001. PMID: 11730318 Review.
-
Comparative study of the molecular mechanisms of oocyte maturation in amphibians.Comp Biochem Physiol B Biochem Mol Biol. 2000 Jun;126(2):189-97. doi: 10.1016/s0305-0491(00)00197-8. Comp Biochem Physiol B Biochem Mol Biol. 2000. PMID: 10874166 Review.
Cited by
-
Characterization of a Protein Phosphatase Type-1 and a Kinase Anchoring Protein in Plasmodium falciparum.Front Microbiol. 2018 Oct 31;9:2617. doi: 10.3389/fmicb.2018.02617. eCollection 2018. Front Microbiol. 2018. PMID: 30429842 Free PMC article.
-
Signal propagation of the MAPK cascade in Xenopus oocytes: role of bistability and ultrasensitivity for a mixed problem.J Math Biol. 2012 Jan;64(1-2):1-39. doi: 10.1007/s00285-011-0403-y. Epub 2011 Feb 3. J Math Biol. 2012. PMID: 21290128
-
A dynamical model of oocyte maturation unveils precisely orchestrated meiotic decisions.PLoS Comput Biol. 2012 Jan;8(1):e1002329. doi: 10.1371/journal.pcbi.1002329. Epub 2012 Jan 5. PLoS Comput Biol. 2012. PMID: 22238511 Free PMC article.
References
-
- Abrieu, A, Doree, M, and Fisher, D (2001). “The interplay between cyclin-B-Cdc2 kinase (MPF) and MAP kinase during maturation of oocytes.” J. Cell Sci. 114, 257–267. - PubMed
-
- Baert, F Y, Bodart, J F, Bocquet-Muchembled, B, Lescuyer-Rousseau, A, and Vilain, J P (2003). “Xp42 (Mpk1) activation is not required for germinal vesicle breakdown but for Raf complete phosphorylation in insulin-stimulated Xenopus oocytes.” J. Biol. Chem. JBCHA3 278, 49714–49720.10.1074/jbc.M308067200 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
