Drosophila morphogenesis: tissue force laws and the modeling of dorsal closure
- PMID: 20514134
- PMCID: PMC2839815
- DOI: 10.2976/1.3266062
Drosophila morphogenesis: tissue force laws and the modeling of dorsal closure
Abstract
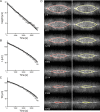
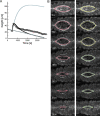
Dorsal closure, a stage of Drosophila development, is a model system for cell sheet morphogenesis and wound healing. During closure, two flanks of epidermal tissue progressively advance to reduce the area of the eye-shaped opening in the dorsal surface, which contains amnioserosa tissue. To simulate the time evolution of the overall shape of the dorsal opening, we developed a mathematical model, in which contractility and elasticity are manifest in model force-producing elements that satisfy force-velocity relationships similar to muscle. The action of the elements is consistent with the force-producing behavior of actin and myosin in cells. The parameters that characterize the simulated embryos were optimized by reference to experimental observations on wild-type embryos and, to a lesser extent, on embryos whose amnioserosa was removed by laser surgery and on myospheroid mutant embryos. Simulations failed to reproduce the amnioserosa-removal protocol in either the elastic or the contractile limit, indicating that both elastic and contractile dynamics are essential components of the biological force-producing elements. We found it was necessary to actively upregulate forces to recapitulate both the double and single-canthus nick protocols, which did not participate in the optimization of parameters, suggesting the existence of additional key feedback mechanisms.
Figures






References
-
- Arfken, G B (1985). “The method of steepest descents.” Mathematical Methods for Physicists, Academic, Orlando, FL, pp. 428–436.
-
- Bloor, J W, and Kiehart, D P (2002). “Drosophila RhoA regulates the cytoskeleton and cell-cell adhesion in the developing epidermis.” Development DEVPED 129, 3173–3183. - PubMed
-
- Colombelli, J, Besser, A, Kress, H, Reynaud, E G, Girard, P, Caussinus, E, Haselmann, U, Small, J V, Schwarz, U S, and Stelzer, E H (2009). “Mechanosensing in actin stress fibers revealed by a close correlation between force and protein localization.” J. Cell Sci. 122, 1665–1679.10.1242/jcs.042986 - DOI - PubMed
-
- See EPAPS Document No. E-HJFOA5-3-008907 for supplemental material. This document can be reached through a direct link in the online’s HTML reference section or via the EPAPS homepage (http://www.aip.org/pubservs/epaps.html).
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
