A new aspect of the TrkB signaling pathway in neural plasticity
- PMID: 20514207
- PMCID: PMC2811861
- DOI: 10.2174/157015909790031210
A new aspect of the TrkB signaling pathway in neural plasticity
Abstract
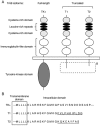
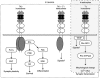
In the central nervous system (CNS), the expression of molecules is strictly regulated during development. Control of the spatiotemporal expression of molecules is a mechanism not only to construct the functional neuronal network but also to adjust the network in response to new information from outside of the individual, i.e., through learning and memory. Among the functional molecules in the CNS, one of the best-studied groups is the neurotrophins, which are nerve growth factor (NGF)-related gene family molecules. Neurotrophins include NGF, brain-derived neurotrophic factor (BDNF), neurotrophin 3 (NT-3), and NT-4/5 in the mammal. Among neurotrophins and their receptors, BDNF and tropomyosin-related kinases B (TrkB) are enriched in the CNS. In the CNS, the BDNF-TrkB signaling pathway fulfills a wide variety of functions throughout life, such as cell survival, migration, outgrowth of axons and dendrites, synaptogenesis, synaptic transmission, and remodeling of synapses. Although the same ligand and receptor, BDNF and TrkB, act in these various developmental events, we do not yet understand what kind of mechanism provokes the functional multiplicity of the BDNF-TrkB signaling pathway. In this review, we discuss the mechanism that elicits the variety of functions performed by the BDNF-TrkB signaling pathway in the CNS as a tool of pharmacological therapy.
Keywords: Brain-derived neurotrophic factor; development; intracellular signaling; morphology; neural plasticity; neuron-glia interaction; receptor dimerization; truncated TrkB-T1..
Figures
Similar articles
-
Truncated trkB receptors on nonneuronal cells inhibit BDNF-induced neurite outgrowth in vitro.Exp Neurol. 1997 Dec;148(2):616-27. doi: 10.1006/exnr.1997.6699. Exp Neurol. 1997. PMID: 9417837
-
Differential dependency of cutaneous mechanoreceptors on neurotrophins, trk receptors, and P75 LNGFR.Dev Biol. 1997 Oct 1;190(1):94-116. doi: 10.1006/dbio.1997.8658. Dev Biol. 1997. PMID: 9331334
-
Neurotrophin-dependent modulation of glutamatergic synaptic transmission in the mammalian CNS.Gen Pharmacol. 1998 Nov;31(5):667-74. doi: 10.1016/s0306-3623(98)00190-6. Gen Pharmacol. 1998. PMID: 9809461 Review.
-
Molecular characterization of neurotrophin expression and the corresponding tropomyosin receptor kinases (trks) in epithelial and stromal cells of the human prostate.Mol Cell Endocrinol. 1997 Oct 31;134(1):15-22. doi: 10.1016/s0303-7207(97)00165-2. Mol Cell Endocrinol. 1997. PMID: 9406845
-
Targeting the BDNF/TrkB pathway for the treatment of tumors.Oncol Lett. 2019 Feb;17(2):2031-2039. doi: 10.3892/ol.2018.9854. Epub 2018 Dec 20. Oncol Lett. 2019. PMID: 30675270 Free PMC article. Review.
Cited by
-
Role of BDNF Signaling in the Neuroprotective and Memory-enhancing Effects of Flavonoids in Alzheimer's Disease.CNS Neurol Disord Drug Targets. 2024;23(8):984-995. doi: 10.2174/1871527323666230912090856. CNS Neurol Disord Drug Targets. 2024. PMID: 37702162 Review.
-
Brain-derived neurotrophic factor in the airways.Pharmacol Ther. 2014 Jul;143(1):74-86. doi: 10.1016/j.pharmthera.2014.02.006. Epub 2014 Feb 19. Pharmacol Ther. 2014. PMID: 24560686 Free PMC article. Review.
-
The responsiveness of TrkB to exogenous BDNF in frontal cortex during antibiotic treatment of Streptococcus pneumoniae meningitis.Neurol Sci. 2014 Dec;35(12):1915-23. doi: 10.1007/s10072-014-1862-x. Epub 2014 Jul 11. Neurol Sci. 2014. PMID: 25012754
-
Deletion of the endogenous TrkB.T1 receptor isoform restores the number of hippocampal CA1 parvalbumin-positive neurons and rescues long-term potentiation in pre-symptomatic mSOD1(G93A) ALS mice.Mol Cell Neurosci. 2018 Jun;89:33-41. doi: 10.1016/j.mcn.2018.03.010. Epub 2018 Mar 24. Mol Cell Neurosci. 2018. PMID: 29580900 Free PMC article.
-
Neuroprotection and Non-Invasive Brain Stimulation: Facts or Fiction?Int J Mol Sci. 2022 Nov 9;23(22):13775. doi: 10.3390/ijms232213775. Int J Mol Sci. 2022. PMID: 36430251 Free PMC article. Review.
References
-
- Aguado F, Carmona MA, Pozas E, Aguiló A, Matrínes-Guijarro FJ, Alcantara S, Borrell V, Yuste R, Ibañes CF, Soriano E. BDNF Regulates Spontaneous Correlated Activity at Early Developmental Stages by Increasing Synaptogenesis and Expression of the K+/Cl- Co-transporter KCC2. Development. 2003;130:1267–1280. - PubMed
-
- Alsina B, Vu T, Cohen-Cory S. Visualizing Synapse Formation in Arborizing Optic Axons in vivo: Dynamics and Modulation by BDNF. Nat. Neurosci. 2001;4:1093–1101. - PubMed
-
- Alter CA, Cai N, Bliven T, Juhansz M, Conner JM, Acheson AL, Lindsay RM, Wiegand SJ. Anterograde Transport of Brain-derived Neurotrophic Factor and Its Role in the Brain. Nature. 1997;389:856–860. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
