Perspectives in cell cycle regulation: lessons from an anoxic vertebrate
- PMID: 20514219
- PMCID: PMC2817888
- DOI: 10.2174/138920209789503905
Perspectives in cell cycle regulation: lessons from an anoxic vertebrate
Abstract
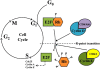
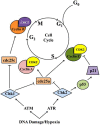
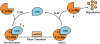
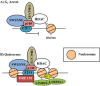
The ability of an animal, normally dependent on aerobic respiration, to suspend breathing and enter an anoxic state for long term survival is clearly a fascinating feat, and has been the focus of numerous biochemical studies. When anoxia tolerant turtles are faced with periods of oxygen deprivation, numerous physiological and biochemical alterations take place in order to facilitate vital reductions in ATP consumption. Such strategies include reversible post-translational modifications as well as the implementation of translation and transcription controls facilitating metabolic depression. Although it is clear that anoxic survival relies on the suppression of ATP consuming processes, the state of the cell cycle in anoxia tolerant vertebrates remain elusive. Several anoxia tolerant invertebrate and embryonic vertebrate models display cell cycle arrest when presented with anoxic stress. Despite this, the cell cycle has not yet been characterized for anoxia tolerant turtles. Understanding how vertebrates respond to anoxia can have important clinical implications. Uncontrollable cellular proliferation and hypoxic tumor progression are inescapably linked in vertebrate tissues. Consequentially, the molecular mechanisms controlling these processes have profound clinical consequences. This review article will discuss the theory of cell cycle arrest in anoxic vertebrates and more specifically, the control of the retinoblastoma pathway, the molecular markers of cell cycle arrest, the activation of checkpoint kinases, and the possibility of translational controls implemented by microRNAs.
Keywords: Cell cycle; Trachemys scripta elegans.; anoxia tolerance; chromatin remodeling; ischemia; microRNA; retinoblastoma.
Figures

References
-
- Lipton P. Ischemic cell death in brain neurons. Physiol. Rev. 1999;17:1431–1568. - PubMed
-
- Bonetta L. Going on a cancer gene hunt. Cell. 2005;123:735–737. - PubMed
-
- Clegg J.S. Embryos of Artemia franciscana survive four years of continuous anoxia: the case for complete metabolic rate depression. J. Exp. Biol. 1997;200:467–475. - PubMed
-
- Hand S.C. Quiescence in Artemia franciscana embryos: reversible arrest of metabolism and gene expression at low oxygen levels. J. Exp. Biol. 1998;201:1233–1242. - PubMed
LinkOut - more resources
Full Text Sources
