Effect of E-cadherin expression on hormone production in rat anterior pituitary lactotrophs in vitro
- PMID: 20514296
- PMCID: PMC2875860
- DOI: 10.1267/ahc.10001
Effect of E-cadherin expression on hormone production in rat anterior pituitary lactotrophs in vitro
Abstract
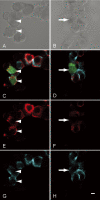
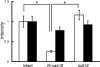
Cadherins are a family of transmembrane glycoproteins that mediate cell-to-cell adhesion. A change in cadherin type in cells, i.e., cadherin switching, induces changes in the character of the cell. Recent studies of the developing rat adenohypophysis found that primordial cells co-expressed E- and N-cadherins, but that hormone-producing cells lost E-cadherin and ultimately possessed only N-cadherin. In the present study, we examined the roles of cadherin switching in cytogenesis of anterior pituitary cells by observing prolactin mRNA and protein expression in lactotrophs that were transformed with an E-cadherin expression vector. In hormone-producing cells that were transfected with a pIRES2-ZsGreen1 plasmid with a full-length E-cadherin cDNA (rE-cad-IZ) insert in primary culture, we detected E- and N-cadherins on plasma membrane and E-cadherin in cytoplasm. In these rE-cad-IZ-transfected cells, in situ hybridization revealed prolactin mRNA signals that were at a level identical to that in control cells, while prolactin protein was barely detectable using immunocytochemistry. The mean signal intensity of prolactin protein in rE-cad-IZ-transfected cells was approximately one fourth that in intact cells and in null-IZ-transfected cells (P<0.01). These results suggest that the expression of E-cadherin does not affect prolactin mRNA transcription; rather, it reduces prolactin protein content, presumably by affecting trafficking of secretory granules.
Keywords: E-cadherin; anterior pituitary; hormone production; prolactin; transfection.
Figures

Similar articles
-
Spatio-temporal relation between cadherin switching and cytogenesis of hormone-producing cells in the developing rat adenohypophysis.Anat Sci Int. 2009 Sep;84(3):155-60. doi: 10.1007/s12565-009-0020-7. Epub 2009 Mar 4. Anat Sci Int. 2009. PMID: 19259769
-
Changes in E- and N-cadherin expression in developing rat adenohypophysis.Anat Rec (Hoboken). 2007 May;290(5):486-90. doi: 10.1002/ar.20516. Anat Rec (Hoboken). 2007. PMID: 17373711
-
Notch signaling-mediated cell-to-cell interaction is dependent on E-cadherin adhesion in adult rat anterior pituitary.Cell Tissue Res. 2017 Apr;368(1):125-133. doi: 10.1007/s00441-016-2540-5. Epub 2016 Dec 10. Cell Tissue Res. 2017. PMID: 27942853
-
A family of POU-domain and Pit-1 tissue-specific transcription factors in pituitary and neuroendocrine development.Annu Rev Physiol. 1990;52:773-91. doi: 10.1146/annurev.ph.52.030190.004013. Annu Rev Physiol. 1990. PMID: 2184776 Review.
-
Pituitary prolactin-secreting tumor formation: recent developments.Biol Signals Recept. 2000 Jan-Feb;9(1):1-20. doi: 10.1159/000014618. Biol Signals Recept. 2000. PMID: 10686432 Review.
Cited by
-
Prognostic Factors for Invasiveness and Recurrence of Pituitary Adenomas: A Series of 94 Patients.Diagnostics (Basel). 2022 Oct 5;12(10):2413. doi: 10.3390/diagnostics12102413. Diagnostics (Basel). 2022. PMID: 36292101 Free PMC article.
-
Compensatory upregulation of myelin protein zero-like 2 expression in spermatogenic cells in cell adhesion molecule-1-deficient mice.Acta Histochem Cytochem. 2012 Feb 29;45(1):47-56. doi: 10.1267/ahc.11057. Epub 2012 Jan 24. Acta Histochem Cytochem. 2012. PMID: 22489104 Free PMC article.
-
Roles of differential expression of microRNA-21-3p and microRNA-433 in FSH regulation in rat anterior pituitary cells.Oncotarget. 2017 May 30;8(22):36553-36565. doi: 10.18632/oncotarget.16615. Oncotarget. 2017. PMID: 28402262 Free PMC article.
-
Live staining and isolation of specific hormone-producing cells from rat anterior pituitary by cytochemistry with lectins and cholera toxin B subunit.Acta Histochem Cytochem. 2011 Aug 27;44(4):159-64. doi: 10.1267/ahc.11016. Epub 2011 Jul 20. Acta Histochem Cytochem. 2011. PMID: 21927514 Free PMC article.
-
FSH Levels Are Related to E-cadherin Expression and Subcellular Location in Nonfunctioning Pituitary Tumors.J Clin Endocrinol Metab. 2020 Aug 1;105(8):2587-94. doi: 10.1210/clinem/dgaa281. J Clin Endocrinol Metab. 2020. PMID: 32421791 Free PMC article.
References
-
- Ben-Jonathan N., Hnasko R. Dopamine as a prolactin (PRL) inhibitor. Endocr. Rev. 2001;22:724–763. - PubMed
-
- Bosco D., Rouiller D. G., Halban P. A. Differential expression of E-cadherin at the surface of rat beta-cells as a marker of functional heterogeneity. J. Endocrinol. 2007;194:21–29. - PubMed
-
- Eastham A. M., Spencer H., Soncin F., Ritson S., Merry C. L., Stern P. L., Ward C. M. Epithelial-mesenchymal transition events during human embryonic stem cell differentiation. Cancer Res. 2007;67:11254–11262. - PubMed
-
- Fujiwara K., Maekawa F., Kikuchi M., Takigami S., Yada T., Yashiro T. Expression of retinaldehyde dehydrogenase (RALDH)2 and RALDH3 but not RALDH1 in the developing anterior pituitary glands of rats. Cell Tissue Res. 2007;328:129–135. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

