The potency of the fs260 connexin43 mutant to impair keratinocyte differentiation is distinct from other disease-linked connexin43 mutants
- PMID: 20515445
- PMCID: PMC2907710
- DOI: 10.1042/BJ20100155
The potency of the fs260 connexin43 mutant to impair keratinocyte differentiation is distinct from other disease-linked connexin43 mutants
Abstract
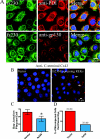
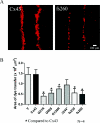
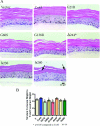
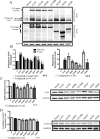
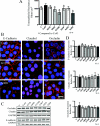
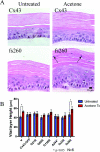
Although there are currently 62 mutants of Cx43 (connexin43) that can cause ODDD (oculodentodigital dysplasia), only two mutants have also been reported to cause palmar plantar hyperkeratosis. To determine how mutants of Cx43 can lead to this skin disease, REKs (rat epidermal keratinocytes) were engineered to express an ODDD-associated Cx43 mutant always linked to skin disease (fs260), an ODDD-linked Cx43 mutant which has been reported to sometimes cause skin disease (fs230), Cx43 mutants which cause ODDD only (G21R, G138R), a mouse Cx43 mutant linked to ODDD (G60S), a non-disease-linked truncated Cx43 mutant that is trapped in the endoplasmic reticulum (Delta244*) or full-length Cx43. When grown in organotypic cultures, of all the mutants investigated, only the fs260-expressing REKs consistently developed a thinner stratum corneum and expressed lower levels of Cx43, Cx26 and loricrin in comparison with REKs overexpressing wild-type Cx43. REKs expressing the fs260 mutant also developed a larger organotypic vital layer after acetone-induced injury and exhibited characteristics of parakeratosis. Collectively, our results suggest that the increased skin disease burden exhibited in ODDD patients harbouring the fs260 mutant is probably due to multiple additive effects cause by the mutant during epidermal differentiation.
Figures

Similar articles
-
Connexin levels regulate keratinocyte differentiation in the epidermis.J Biol Chem. 2007 Oct 12;282(41):30171-80. doi: 10.1074/jbc.M703623200. Epub 2007 Aug 10. J Biol Chem. 2007. PMID: 17693411
-
Functional characterization of a GJA1 frameshift mutation causing oculodentodigital dysplasia and palmoplantar keratoderma.J Biol Chem. 2006 Oct 20;281(42):31801-11. doi: 10.1074/jbc.M605961200. Epub 2006 Aug 6. J Biol Chem. 2006. PMID: 16891658
-
Differential potency of dominant negative connexin43 mutants in oculodentodigital dysplasia.J Biol Chem. 2007 Jun 29;282(26):19190-202. doi: 10.1074/jbc.M609653200. Epub 2007 Apr 9. J Biol Chem. 2007. PMID: 17420259
-
Astrocytic Connexin43 Channels as Candidate Targets in Epilepsy Treatment.Biomolecules. 2020 Nov 20;10(11):1578. doi: 10.3390/biom10111578. Biomolecules. 2020. PMID: 33233647 Free PMC article. Review.
-
Expression and function of connexins in the epidermis, analyzed with transgenic mouse mutants.Eur J Cell Biol. 2004 Dec;83(11-12):647-54. doi: 10.1078/0171-9335-00422. Eur J Cell Biol. 2004. PMID: 15679109 Review.
Cited by
-
Role of connexins and pannexins during ontogeny, regeneration, and pathologies of bone.BMC Cell Biol. 2016 May 24;17 Suppl 1(Suppl 1):19. doi: 10.1186/s12860-016-0088-6. BMC Cell Biol. 2016. PMID: 27230612 Free PMC article. Review.
-
Human dermal fibroblasts derived from oculodentodigital dysplasia patients suggest that patients may have wound-healing defects.Hum Mutat. 2011 Apr;32(4):456-66. doi: 10.1002/humu.21472. Hum Mutat. 2011. PMID: 21305658 Free PMC article.
-
Connexinopathies: a structural and functional glimpse.BMC Cell Biol. 2016 May 24;17 Suppl 1(Suppl 1):17. doi: 10.1186/s12860-016-0092-x. BMC Cell Biol. 2016. PMID: 27228968 Free PMC article. Review.
-
Phosphorylation on Ser-279 and Ser-282 of connexin43 regulates endocytosis and gap junction assembly in pancreatic cancer cells.Mol Biol Cell. 2013 Mar;24(6):715-33. doi: 10.1091/mbc.E12-07-0537. Epub 2013 Jan 30. Mol Biol Cell. 2013. PMID: 23363606 Free PMC article.
-
Myogenic bladder defects in mouse models of human oculodentodigital dysplasia.Biochem J. 2014 Feb 1;457(3):441-9. doi: 10.1042/BJ20130810. Biochem J. 2014. PMID: 24228978 Free PMC article.
References
-
- Goodenough D. A., Goliger J. A., Paul D. L. Connexins, connexons, and intercellular communication. Annu. Rev. Biochem. 1996;65:475–502. - PubMed
-
- Gong X. Q., Shao Q., Lounsbury C. S., Bai D., Laird D. W. Functional characterization of a GJA1 frameshift mutation causing oculodentodigital dysplasia and palmoplantar keratoderma. J. Biol. Chem. 2006;281:31801–31811. - PubMed
-
- Lai A., Le D. N., Paznekas W. A., Gifford W. D., Jabs E. W., Charles A. C. Oculodentodigital dysplasia connexin43 mutations result in non-functional connexin hemichannels and gap junctions in C6 glioma cells. J. Cell Sci. 2006;119:532–541. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

