Surveillance of active human cytomegalovirus infection in hematopoietic stem cell transplantation (HLA sibling identical donor): search for optimal cutoff value by real-time PCR
- PMID: 20515464
- PMCID: PMC2890007
- DOI: 10.1186/1471-2334-10-147
Surveillance of active human cytomegalovirus infection in hematopoietic stem cell transplantation (HLA sibling identical donor): search for optimal cutoff value by real-time PCR
Abstract
Background: Human cytomegalovirus (CMV) infection still causes significant morbidity and mortality after allogeneic hematopoietic stem cell transplantation (HSCT). Therefore, it is extremely important to diagnosis and monitor active CMV infection in HSCT patients, defining the CMV DNA levels of virus replication that warrant intervention with antiviral agents in order to accurately prevent CMV disease and further related complications.
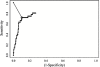
Methods: During the first 150 days after allogeneic HSTC, thirty patients were monitored weekly for active CMV infection by pp65 antigenemia, nested-PCR and real-time PCR assays. Receiver operating characteristic (ROC) plot analysis was performed to determine a threshold value of the CMV DNA load by real-time PCR.
Results: Using ROC curves, the optimal cutoff value by real-time PCR was 418.4 copies/104 PBL (sensitivity, 71.4%; specificity, 89.7%). Twenty seven (90%) of the 30 analyzed patients had active CMV infection and two (6.7%) developed CMV disease. Eleven (40.7%) of these 27 patients had acute GVHD, 18 (66.7%) had opportunistic infection, 5 (18.5%) had chronic rejection and 11 (40.7%) died - one died of CMV disease associated with GVHD and bacterial infection.
Conclusions: The low incidence of CMV disease in HSCT recipients in our study attests to the efficacy of CMV surveillance based on clinical routine assay. The quantification of CMV DNA load using real-time PCR appears to be applicable to the clinical practice and an optimal cutoff value for guiding timely preemptive therapy should be clinically validated in future studies.
Figures
References
-
- Nichols WG, Corey L, Gooley T, Davis C, Boeckh M. High risk of death due to bacterial and fungal infection among cytomegalovirus (CMV)-seronegative recipients of stem cell transplants from seropositive donors: evidence for indirect effects of primary CMV infection. J Infect Dis. 2002;185:273–282. doi: 10.1086/338624. - DOI - PubMed
-
- Machida U, Kami M, Fukui T, Kazuyama Y, Kinoshita M, Tanaka Kanda Y, Ogawa S, Honda H, Chiba S, Mitani K, Muto Y, Osumi K, Kimura S, Hirai H. Real-Time automated PCR for early diagnosis and monitoring of cytomegalovirus infection after bone marrow transplantation. J Clin Microbiol. 2000;38:2536–2542. - PMC - PubMed
-
- Boeckh M, Leisenring W, Riddell SR, Bowden RA, Huang ML, Myerson D, Stevens-Ayers T, Flowers ME, Cunningham T, Corey L. Late cytomegalovirus disease and mortality in recipients of allogeneic hematopoietic stem cell transplants: importance of viral load and T-cell immunity. Blood. 2003;101:407–414. doi: 10.1182/blood-2002-03-0993. - DOI - PubMed
-
- Laso JF. Diagnostico Diferencial en Medicina Interna. 2. Barcelona: Elsevier Espanã; 2005. p. 497.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

