A prospective randomised trial comparing nasogastric with intravenous hydration in children with bronchiolitis (protocol): the comparative rehydration in bronchiolitis study (CRIB)
- PMID: 20515467
- PMCID: PMC2903564
- DOI: 10.1186/1471-2431-10-37
A prospective randomised trial comparing nasogastric with intravenous hydration in children with bronchiolitis (protocol): the comparative rehydration in bronchiolitis study (CRIB)
Abstract
Background: Bronchiolitis is the most common reason for admission of infants to hospital in developed countries. Fluid replacement therapy is required in about 30% of children admitted with bronchiolitis. There are currently two techniques of fluid replacement therapy that are used with the same frequency-intravenous (IV) or nasogastric (NG).The evidence to determine the optimum route of hydration therapy for infants with bronchiolitis is inadequate. This randomised trial will be the first to provide good quality evidence of whether nasogastric rehydration (NGR) offers benefits over intravenous rehydration (IVR) using the clinically relevant continuous outcome measure of duration of hospital admission.
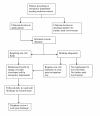
Methods/design: A prospective randomised multi-centre trial in Australia and New Zealand where children between 2 and 12 months of age with bronchiolitis, needing non oral fluid replacement, are randomised to receive either intravenous (IV) or nasogastric (NG) rehydration.750 patients admitted to participating hospitals will be recruited, and will be followed daily during the admission and by telephone 1 week after discharge. Patients with chronic respiratory, cardiac, or neurological disease; choanal atresia; needing IV fluid resuscitation; needing an IV for other reasons, and those requiring CPAP or ventilation are excluded.The primary endpoint is duration of hospital admission. Secondary outcomes are complications, need for ICU admission, parental satisfaction, and an economic evaluation. Results will be analysed using t-test for continuous data, and chi squared for categorical data. Non parametric data will be log transformed.
Discussion: This trial will define the role of NGR and IVR in bronchiolitis
Trial registration: The trial is registered with the Australian and New Zealand Clinical Trials Registry--ACTRN12605000033640.
Figures
References
-
- Bush A, Thomson AH. Acute bronchiolitis.[see comment] BMJ. 2007;335(7628):1037–1041. doi: 10.1136/bmj.39374.600081.AD. - DOI - PMC - PubMed
-
- Leader S, Kolhase K. Recent trends in severe respiratory syncytial virus (RSV) among US infants, 1997-2000. J Pediatr. 2003;143(5 suppl):s127–s132. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources