Th 17 cells interplay with Foxp3+ Tregs in regulation of inflammation and autoimmunity
- PMID: 20515737
- PMCID: PMC2880832
- DOI: 10.2741/3657
Th 17 cells interplay with Foxp3+ Tregs in regulation of inflammation and autoimmunity
Abstract
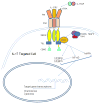
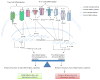
T helper 17 cells (Th17) are a new CD4+ T helper subset that has been implicated in inflammatory and autoimmune diseases. Th17, along with CD4(+)CD25(high) Foxp3(+) regulatory T cells (Tregs) and other new T helper subsets, have expanded the Th1-Th2 paradigm. Although this new eight-subset paradigm significantly improved our understanding on the differentiation and regulation of CD4+ T helper subsets, many questions remain to be answered. Here we will briefly review the following issues: a) Old Th1-Th2 paradigm versus new multi-subset paradigm; b) Structural features of IL-17 family cytokines; c) Th17 cells; d) Effects of IL-17 on various cell types and tissues; e) IL-17 receptor and signaling pathways; f) Th17-mediated inflammations; and g) Protective mechanisms of IL-17 in infections. Lastly, we will examine the interactions of Th17 and Treg in autoimmune diseases and inflammation: Th17 cells interplay with Tregs. Regulation of autoimmunity and inflammation lies in the interplays of the different T helper subsets, therefore, better understanding of these subsets' interactions would greatly improve our approaches in developing therapy to combat inflammatory and autoimmune diseases.
Figures


References
-
- Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. - PubMed
-
- Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Aging-induced proinflammatory shift in cytokine expression profile in coronary arteries. Faseb J. 2003;17:1183–5. - PubMed
-
- de Boer OJ, van der Meer JJ, Teeling P, van der Loos CM, Idu MM, van Maldegem F, Aten J, van der Wal AC. Differential expression of interleukin-17 family cytokines in intact and complicated human atherosclerotic plaques. J pathol. 2009 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

