Complement factor H autoantibodies are associated with early stage NSCLC
- PMID: 20515868
- PMCID: PMC2891404
- DOI: 10.1158/1078-0432.CCR-10-0321
Complement factor H autoantibodies are associated with early stage NSCLC
Abstract
Purpose: To discover diagnostic biomarkers associated with early-stage non-small cell lung cancer (NSCLC), we searched for autoantibodies preferentially present in stage I patients compared with patients with advanced-stage disease. Here we describe an autoantibody against complement factor H (CFH) and this autoantibody's association with early-stage NSCLC.
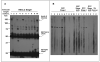
Experimental design: Immunoblots were used to detect autoantibodies in the sera of stage I NSCLC patients. An autoantibody recognizing a 150 kDa protein was discovered, and the protein was identified by mass spectrometry. The association of the autoantibody with early-stage disease was suggested by the results of immunoblot analysis with sera from 28 stage I patients and 28 stage III/IV patients. This association was confirmed by protein microarray of sera from 125 NSCLC patients of all stages as well as 125 controls matched by age, gender, and smoking history.
Results: The immunoreactive protein was identified as CFH. By immunoblot analysis, anti-CFH autoantibody was found in 50% of stage I NSCLC patients and 11% of late-stage NSCLC patients (P = 0.003). By protein microarray analysis, patients with stage I NSCLC had a significantly higher incidence of anti-CFH antibody than those with late-stage NSCLC (P = 0.0051). The percentage of sera with a positive level of CFH autoantibody was 30.4% in stage I, 21.1% in stage II, 12.5% in stage III, 7.4% in stage IV, and 8.0% in the control group.
Conclusions: These findings suggest that in patients with NSCLC, CFH autoantibody is a molecular marker associated with early-stage disease.
(c) 2010 AACR.
Figures



References
-
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2:706–14. - PubMed
-
- Groome PA, Bolejack V, Crowley JJ, et al. The IASLC Lung Cancer Staging Project: validation of the proposals for revision of the T, N, and M descriptors and consequent stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007;2:694–705. - PubMed
-
- Hoagland LF, 4th, Campa MJ, Gottlin EB, Herndon JE, 2nd, Patz EF., Jr Haptoglobin and posttranslational glycan-modified derivatives as serum biomarkers for the diagnosis of nonsmall cell lung cancer. Cancer. 2007;110:2260–8. - PubMed
-
- Sayegh R, Awwad JT, Maxwell C, Lessey B, Isaacson K. Alpha 2-macroglobulin production by the human endometrium. J Clin Endocrinol Metab. 1995;80:1021–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

