Coxiella burnetii phase I and II variants replicate with similar kinetics in degradative phagolysosome-like compartments of human macrophages
- PMID: 20515926
- PMCID: PMC2916283
- DOI: 10.1128/IAI.00406-10
Coxiella burnetii phase I and II variants replicate with similar kinetics in degradative phagolysosome-like compartments of human macrophages
Abstract
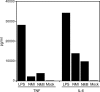
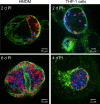
Coxiella burnetii infects mononuclear phagocytes, where it directs biogenesis of a vacuolar niche termed the parasitophorous vacuole (PV). Owing to its lumenal pH (approximately 5) and fusion with endolysosomal vesicles, the PV is considered phagolysosome-like. However, the degradative properties of the mature PV are unknown, and there are conflicting reports on the maturation state and growth permissiveness of PV harboring virulent phase I or avirulent phase II C. burnetii variants in human mononuclear phagocytes. Here, we employed infection of primary human monocyte-derived macrophages (HMDMs) and THP-1 cells as host cells to directly compare the PV maturation kinetics and pathogen growth in cells infected with the Nine Mile phase I variant (NMI) or phase II variant (NMII) of C. burnetii. In both cell types, phase variants replicated with similar kinetics, achieving roughly 2 to 3 log units of growth before they reached stationary phase. HMDMs infected by either phase variant secreted similar amounts of the proinflammatory cytokines interleukin-6 and tumor necrosis factor alpha. In infected THP-1 cells, equal percentages of NMI and NMII PVs decorate with the early endosomal marker Rab5, the late endosomal/lysosomal markers Rab7 and CD63, and the lysosomal marker cathepsin D at early (8 h) and late (72 h) time points postinfection (p.i.). Mature PVs (2 to 4 days p.i.) harboring NMI or NMII contained proteolytically active cathepsins and quickly degraded Escherichia coli. These data suggest that C. burnetii does not actively inhibit phagolysosome function as a survival mechanism. Instead, NMI and NMII resist degradation to replicate in indistinguishable digestive PVs that fully mature through the endolysosomal pathway.
Figures



References
-
- Andoh, M., G. Zhang, K. E. Russell-Lodrigue, H. R. Shive, B. R. Weeks, and J. E. Samuel. 2007. T cells are essential for bacterial clearance, and gamma interferon, tumor necrosis factor alpha, and B cells are crucial for disease development in Coxiella burnetii infection in mice. Infect. Immun. 75:3245-3255. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

