Antiangiogenic cancer therapy: monitoring with molecular US and a clinically translatable contrast agent (BR55)
- PMID: 20515975
- PMCID: PMC2909432
- DOI: 10.1148/radiol.10091858
Antiangiogenic cancer therapy: monitoring with molecular US and a clinically translatable contrast agent (BR55)
Abstract
Purpose: To develop and test human kinase insert domain receptor (KDR)-targeted microbubbles (MBs) (MB(KDR)) for imaging KDR at the molecular level and for monitoring antiangiogenic therapy in a human colon cancer xenograft tumor model in mice.
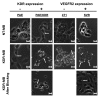
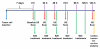
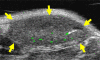
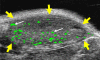
Materials and methods: Animal studies were approved by the Institutional Administrative Panel on Laboratory Animal Care. A heterodimeric peptide that binds to human KDR with low nanomolar affinity (K(D) = 0.5 nmol/L) was coupled onto the surface of perfluorobutane-containing lipid-shelled MBs (MB(KDR)). Binding specificity of MB(KDR) to human KDR and cross-reactivity with murine vascular endothelial growth factor (VEGF) receptor 2 (VEGFR2) were tested in cell culture under flow shear stress conditions (at 100 sec(-1)). In vivo binding specificity of MB(KDR) to VEGFR2 was tested in human LS174T colon cancer xenografts in mice with a 40-MHz ultrasonographic (US) transducer. Targeted contrast material-enhanced US imaging signal by using MB(KDR) was longitudinally measured during 6 days in tumors with (n = 6) and without (n = 6) antiangiogenic treatment (anti-VEGF antibody). Ex vivo VEGFR2 staining and microvessel density analysis were performed. Significant differences were evaluated (t, Mann-Whitney, or Wilcoxon test).
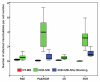
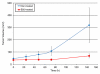
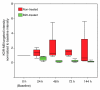
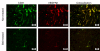
Results: Cell culture experiments showed four times greater binding specificity of MB(KDR) to human KDR and cross-reactivity to murine VEGFR2 (P < or = .01). In vivo imaging signal was more than three times higher (P = .01) with MB(KDR) compared with control MBs and decreased significantly (approximately fourfold lower, P = .03) following in vivo receptor blocking with anti-VEGFR2 antibody. One day after initiation of antiangiogenic therapy, imaging signal was significantly decreased (approximately 46% lower, P = .02) in treated versus untreated tumors; it remained significantly lower (range, 46%-84% decreased; P = .038) during the following 5 days. Microvessel density was significantly reduced (P = .04) in treated (mean, 7.3 microvessels per square millimeter +/- 4.7 [standard deviation]) versus untreated tumors (mean, 22.0 microvessels per square millimeter +/- 9.4); VEGFR2 expression was significantly decreased (>50% lower, P = .03) in treated tumors.
Conclusion: Human MB(KDR) allow in vivo imaging and longitudinal monitoring of VEGFR2 expression in human colon cancer xenografts.
Conflict of interest statement
See Materials and Methods for pertinent disclosures.
Figures

Comment in
-
Science to practice: the dawn of molecular US imaging for clinical cancer imaging.Radiology. 2010 Aug;256(2):331-3. doi: 10.1148/radiol.100717. Radiology. 2010. PMID: 20656826
References
-
- Folkman J. Angiogenesis. Annu Rev Med 2006;57:1–18 - PubMed
-
- Mabille M, Vanel D, Albiter M, et al. Follow-up of hepatic and peritoneal metastases of gastrointestinal tumors (GIST) under Imatinib therapy requires different criteria of radiological evaluation (size is not everything!!!). Eur J Radiol 2009;69(2):204–208 - PubMed
-
- Lindner JR. Microbubbles in medical imaging: current applications and future directions. Nat Rev Drug Discov 2004;3(6):527–532 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

