The role of interchain heterodisulfide formation in activation of the human common beta and mouse betaIL-3 receptors
- PMID: 20516062
- PMCID: PMC2915712
- DOI: 10.1074/jbc.M109.097881
The role of interchain heterodisulfide formation in activation of the human common beta and mouse betaIL-3 receptors
Abstract
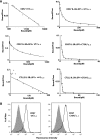
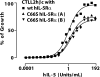
The cytokines, interleukin-3 (IL-3), interleukin-5 (IL-5), and granulocyte-macrophage colony-stimulating factor (GM-CSF), exhibit overlapping activities in the regulation of hematopoietic cells. In humans, the common beta (betac) receptor is shared by the three cytokines and functions together with cytokine-specific alpha subunits in signaling. A widely accepted hypothesis is that receptor activation requires heterodisulfide formation between the domain 1 D-E loop disulfide in human betac (hbetac) and unidentified cysteine residues in the N-terminal domains of the alpha receptors. Since the development of this hypothesis, new data have been obtained showing that domain 1 of hbetac is part of the cytokine binding epitope of this receptor and that an IL-3Ralpha isoform lacking the N-terminal Ig-like domain (the "SP2" isoform) is competent for signaling. We therefore investigated whether distortion of the domain 1-domain 4 ligand-binding epitope in hbetac and the related mouse receptor, beta(IL-3), could account for the loss of receptor signaling when the domain 1 D-E loop disulfide is disrupted. Indeed, mutation of the disulfide in hbetac led to both a complete loss of high affinity binding with the human IL-3Ralpha SP2 isoform and of downstream signaling. Mutation of the orthologous residues in the mouse IL-3-specific receptor, beta(IL-3), not only precluded direct binding of mouse IL-3 but also resulted in complete loss of high affinity binding and signaling with the mouse IL-3Ralpha SP2 isoform. Our data are most consistent with a role for the domain 1 D-E loop disulfide of hbetac and beta(IL-3) in maintaining the precise positions of ligand-binding residues necessary for normal high affinity binding and signaling.
Figures
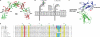
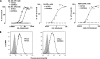
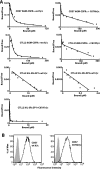
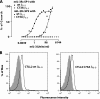
References
-
- Kopf M., Brombacher F., Hodgkin P. D., Ramsay A. J., Milbourne E. A., Dai W. J., Ovington K. S., Behm C. A., Köhler G., Young I. G., Matthaei K. I. (1996) Immunity 4, 15–24 - PubMed
-
- Lantz C. S., Boesiger J., Song C. H., Mach N., Kobayashi T., Mulligan R. C., Nawa Y., Dranoff G., Galli S. J. (1998) Nature 392, 90–93 - PubMed
-
- Hamilton J. A. (2008) Nat. Rev. Immunol. 8, 533–544 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

