Trio is a key guanine nucleotide exchange factor coordinating regulation of the migration and morphogenesis of granule cells in the developing cerebellum
- PMID: 20516067
- PMCID: PMC2915719
- DOI: 10.1074/jbc.M109.096537
Trio is a key guanine nucleotide exchange factor coordinating regulation of the migration and morphogenesis of granule cells in the developing cerebellum
Abstract
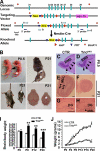
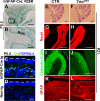
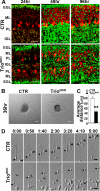
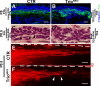
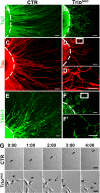
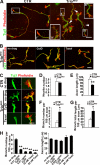
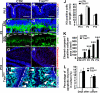
Orchestrated regulation of neuronal migration and morphogenesis is critical for neuronal development and establishment of functional circuits, but its regulatory mechanism is incompletely defined. We established and analyzed mice with neural-specific knock-out of Trio, a guanine nucleotide exchange factor with multiple guanine nucleotide exchange factor domains. Knock-out mice showed defective cerebella and severe signs of ataxia. Mutant cerebella had no granule cells in the internal granule cell layer due to aberrant granule cell migration as well as abnormal neurite growth. Trio-deficient granule cells showed reduced extension of neurites and highly branched and misguided processes with perturbed stabilization of actin and microtubules. Trio deletion caused down-regulation of the activation of Rac1, RhoA, and Cdc42, and mutant granule cells appeared to be unresponsive to neurite growth-promoting molecules such as Netrin-1 and Semaphorin 6A. These results suggest that Trio may be a key signal module for the orchestrated regulation of neuronal migration and morphogenesis during cerebellar development. Trio may serve as a signal integrator decoding extrinsic signals to Rho GTPases for cytoskeleton organization.
Figures

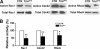
Similar articles
-
Distinct functions of Trio GEF domains in axon outgrowth of cerebellar granule neurons.J Genet Genomics. 2019 Feb;46(2):87-96. doi: 10.1016/j.jgg.2019.02.003. Epub 2019 Feb 23. J Genet Genomics. 2019. PMID: 30850274
-
Golgi-resident TRIO regulates membrane trafficking during neurite outgrowth.J Biol Chem. 2019 Jul 12;294(28):10954-10968. doi: 10.1074/jbc.RA118.007318. Epub 2019 May 31. J Biol Chem. 2019. PMID: 31152060 Free PMC article.
-
Trio mediates netrin-1-induced Rac1 activation in axon outgrowth and guidance.Mol Cell Biol. 2008 Apr;28(7):2314-23. doi: 10.1128/MCB.00998-07. Epub 2008 Jan 22. Mol Cell Biol. 2008. PMID: 18212043 Free PMC article.
-
The Trio family of guanine-nucleotide-exchange factors: regulators of axon guidance.J Cell Sci. 2001 Jun;114(Pt 11):1973-80. doi: 10.1242/jcs.114.11.1973. J Cell Sci. 2001. PMID: 11493634 Review.
-
The guanine nucleotide exchange factor Tiam1: a Janus-faced molecule in cellular signaling.Cell Signal. 2014 Mar;26(3):483-91. doi: 10.1016/j.cellsig.2013.11.034. Epub 2013 Dec 2. Cell Signal. 2014. PMID: 24308970 Review.
Cited by
-
Candidate Genes Associated With Neurological Findings in a Patient With Trisomy 4p16.3 and Monosomy 5p15.2.Front Genet. 2020 Jun 17;11:561. doi: 10.3389/fgene.2020.00561. eCollection 2020. Front Genet. 2020. PMID: 32625234 Free PMC article.
-
Upregulated TRIO expression correlates with a malignant phenotype in human hepatocellular carcinoma.Tumour Biol. 2015 Sep;36(9):6901-8. doi: 10.1007/s13277-015-3377-3. Epub 2015 Apr 8. Tumour Biol. 2015. PMID: 25851347
-
Trio family proteins as regulators of cell migration and morphogenesis in development and disease - mechanisms and cellular contexts.J Cell Sci. 2021 Feb 10;134(3):jcs248393. doi: 10.1242/jcs.248393. J Cell Sci. 2021. PMID: 33568469 Free PMC article. Review.
-
Rac-maninoff and Rho-vel: The symphony of Rho-GTPase signaling at excitatory synapses.Small GTPases. 2022 Jan;13(1):14-47. doi: 10.1080/21541248.2021.1885264. Epub 2021 May 6. Small GTPases. 2022. PMID: 33955328 Free PMC article.
-
The N-terminal DH-PH domain of Trio induces cell spreading and migration by regulating lamellipodia dynamics in a Rac1-dependent fashion.PLoS One. 2012;7(1):e29912. doi: 10.1371/journal.pone.0029912. Epub 2012 Jan 6. PLoS One. 2012. PMID: 22238672 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

