Differences in the large extracellular loop between the K(+)-Cl(-) cotransporters KCC2 and KCC4
- PMID: 20516068
- PMCID: PMC2911324
- DOI: 10.1074/jbc.M110.144063
Differences in the large extracellular loop between the K(+)-Cl(-) cotransporters KCC2 and KCC4
Abstract
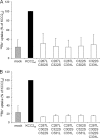
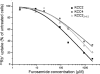
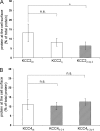
K(+)Cl(-) cotransporters (KCCs) play fundamental physiological roles in processes such as inhibitory neurotransmission and cell volume regulation. Mammalian genomes encode four distinct KCC paralogs, which share basic transport characteristics but differ significantly in ion affinity, pharmacology, and relative sensitivity to cell volume. Studies to identify divergence in functional characteristics have thus far focused on the cytoplasmic termini. Here, we investigated sequence requirements of the large extracellular loop (LEL) for function in KCC2 and KCC4. Mutation of all four evolutionarily conserved cysteines abolished KCC2 transport activity. This behavior differs from that of its closest relative, KCC4, which is insensitive to this mutation. Chimeras supported the differences in the LEL of the two cotransporters, because swapping wild-type LEL resulted in functional KCC2 but rendered KCC4 inactive. Insertion of the quadruple cysteine substitution mutant of the KCC4 loop, which was functional in the parental isoform, abolished transport activity in KCC2. Dose-response curves of wild-type and chimeric KCCs revealed that the LEL contributes to the different sensitivity to loop diuretics; a KCC2 chimera containing the KCC4 LEL displayed an IC(50) of 396.5 mum for furosemide, which was closer to KCC4 (548.8 mum) than to KCC2 (184.4 mum). Cell surface labeling and immunocytochemistry indicated that mutations do not affect trafficking to the plasma membrane. Taken together, our results show a dramatic and unexpected difference in the sequence requirements of the LEL between the closely related KCC2 and KCC4. Furthermore, they demonstrate that evolutionarily highly conserved amino acids can have different functions within KCC members.
Figures

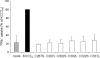



Similar articles
-
Highly conserved ion binding sites are not all functionally relevant in mouse KCC4.Front Mol Biosci. 2025 Mar 31;12:1556250. doi: 10.3389/fmolb.2025.1556250. eCollection 2025. Front Mol Biosci. 2025. PMID: 40230454 Free PMC article.
-
A C-terminal domain in KCC2 confers constitutive K+-Cl- cotransport.J Biol Chem. 2006 Jan 13;281(2):1016-26. doi: 10.1074/jbc.M509972200. Epub 2005 Nov 16. J Biol Chem. 2006. PMID: 16291749
-
Functional comparison of the K+-Cl- cotransporters KCC1 and KCC4.J Biol Chem. 2000 Sep 29;275(39):30326-34. doi: 10.1074/jbc.M003112200. J Biol Chem. 2000. PMID: 10913127
-
[Pathophysiological aspects of K+: Cl- cotransporters].Rev Invest Clin. 2014 Mar-Apr;66(2):173-80. Rev Invest Clin. 2014. PMID: 24960328 Review. Spanish.
-
High-Resolution Views and Transport Mechanisms of the NKCC1 and KCC Transporters.J Mol Biol. 2021 Aug 6;433(16):167056. doi: 10.1016/j.jmb.2021.167056. Epub 2021 May 20. J Mol Biol. 2021. PMID: 34022207 Free PMC article. Review.
Cited by
-
Cryo-EM structures of DrNKCC1 and hKCC1: a new milestone in the physiology of cation-chloride cotransporters.Am J Physiol Cell Physiol. 2020 Feb 1;318(2):C225-C237. doi: 10.1152/ajpcell.00465.2019. Epub 2019 Nov 20. Am J Physiol Cell Physiol. 2020. PMID: 31747317 Free PMC article.
-
K+/Cl- cotransporter 2 (KCC2) and Na+/ cotransporter 1 (NBCe1) interaction modulates profile of KCC2 phosphorylation.Front Cell Neurosci. 2023 Oct 10;17:1253424. doi: 10.3389/fncel.2023.1253424. eCollection 2023. Front Cell Neurosci. 2023. PMID: 37881493 Free PMC article.
-
Highly conserved ion binding sites are not all functionally relevant in mouse KCC4.Front Mol Biosci. 2025 Mar 31;12:1556250. doi: 10.3389/fmolb.2025.1556250. eCollection 2025. Front Mol Biosci. 2025. PMID: 40230454 Free PMC article.
-
Impaired neuronal KCC2 function by biallelic SLC12A5 mutations in migrating focal seizures and severe developmental delay.Sci Rep. 2016 Jul 20;6:30072. doi: 10.1038/srep30072. Sci Rep. 2016. PMID: 27436767 Free PMC article.
-
The biogenesis of potassium transporters: implications of disease-associated mutations.Crit Rev Biochem Mol Biol. 2024 Jun-Aug;59(3-4):154-198. doi: 10.1080/10409238.2024.2369986. Epub 2024 Jul 1. Crit Rev Biochem Mol Biol. 2024. PMID: 38946646 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

