Pannexin1 and Pannexin2 channels show quaternary similarities to connexons and different oligomerization numbers from each other
- PMID: 20516070
- PMCID: PMC2915678
- DOI: 10.1074/jbc.M110.115444
Pannexin1 and Pannexin2 channels show quaternary similarities to connexons and different oligomerization numbers from each other
Abstract
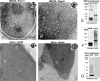
Pannexins are homologous to innexins, the invertebrate gap junction family. However, mammalian pannexin1 does not form canonical gap junctions, instead forming hexameric oligomers in single plasma membranes and intracellularly. Pannexin1 acts as an ATP release channel, whereas less is known about the function of Pannexin2. We purified cellular membranes isolated from MDCK cells stably expressing rat Pannexin1 or Pannexin2 and identified pannexin channels (pannexons) in single membranes by negative stain and immunogold labeling. Protein gel and Western blot analysis confirmed Pannexin1 (Panx1) or Pannexin2 (Panx2) as the channel-forming proteins. We expressed and purified Panx1 and Panx2 using a baculovirus Sf9 expression system and obtained doughnut-like structures similar to those seen previously in purified connexin hemichannels (connexons) and mammalian membranes. Purified pannexons were comparable in size and overall appearance to Connexin46 and Connexin50 connexons. Pannexons and connexons were further analyzed by single-particle averaging for oligomer and pore diameters. The oligomer diameter increased with increasing monomer molecular mass, and we found that the measured oligomeric pore diameter for Panxs was larger than for Connexin26. Panx1 and Panx2 formed active homomeric channels in Xenopus oocytes and in vitro vesicle assays. Cross-linking and native gels of purified homomeric full-length and a C-terminal Panx2 truncation mutant showed a banding pattern more consistent with an octamer. We purified Panx1/Panx2 heteromeric channels and found that they were unstable over time, possibly because Panx1 and Panx2 homomeric pannexons have different monomer sizes and oligomeric symmetry from each other.
Figures






References
-
- D'hondt C., Ponsaerts R., De Smedt H., Bultynck G., Himpens B. (2009) Bioessays 31, 953–974 - PubMed
-
- Panchin Y. V. (2005) J. Exp. Biol. 208, 1415–1419 - PubMed
-
- Penuela S., Bhalla R., Gong X. Q., Cowan K. N., Celetti S. J., Cowan B. J., Bai D., Shao Q., Laird D. W. (2007) J. Cell Sci. 120, 3772–3783 - PubMed
-
- Baranova A., Ivanov D., Petrash N., Pestova A., Skoblov M., Kelmanson I., Shagin D., Nazarenko S., Geraymovych E., Litvin O., Tiunova A., Born T. L., Usman N., Staroverov D., Lukyanov S., Panchin Y. (2004) Genomics 83, 706–716 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

