Differential roles of Epac in regulating cell death in neuronal and myocardial cells
- PMID: 20516079
- PMCID: PMC2911347
- DOI: 10.1074/jbc.M109.094581
Differential roles of Epac in regulating cell death in neuronal and myocardial cells
Abstract
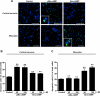
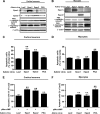
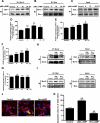
Cell survival and death play critical roles in tissues composed of post-mitotic cells. Cyclic AMP (cAMP) has been known to exert a distinct effect on cell susceptibility to apoptosis, protecting neuronal cells and deteriorating myocardial cells. These effects are primarily studied using protein kinase A activation. In this study we show the differential roles of Epac, an exchange protein activated by cAMP and a new effector molecule of cAMP signaling, in regulating apoptosis in these cell types. Both stimulation of Epac by 8-p-methoxyphenylthon-2'-O-methyl-cAMP and overexpression of Epac significantly increased DNA fragmentation and TUNEL (terminal deoxynucleotidyltransferase-mediated biotin nick end-labeling)-positive cell counts in mouse cortical neurons but not in cardiac myocytes. In contrast, stimulation of protein kinase A increased apoptosis in cardiac myocytes but not in neuronal cells. In cortical neurons the expression of the Bcl-2 interacting member protein (Bim) was increased by stimulation of Epac at the transcriptional level and was decreased in mice with genetic disruption of Epac1. Epac-induced neuronal apoptosis was attenuated by the silencing of Bim. Furthermore, Epac1 disruption in vivo abolished the 3-nitropropionic acid-induced neuronal apoptosis that occurs in wild-type mice. These results suggest that Epac induces neuron-specific apoptosis through increasing Bim expression. Because the disruption of Epac exerted a protective effect on neuronal apoptosis in vivo, the inhibition of Epac may be a consideration in designing a therapeutic strategy for the treatment of neurodegenerative diseases.
Figures






References
-
- Mattson M. P., Kroemer G. (2003) Trends Mol. Med. 9, 196–205 - PubMed
-
- Kobayashi Y., Shinozawa T. (1997) Brain Res. 778, 309–317 - PubMed
-
- Parvathenani L. K., Calandra V., Roberts S. B., Posmantur R. (2000) Neuroreport 11, 2293–2297 - PubMed
-
- Jonakait G. M., Ni L. (2009) Brain Res. 1285, 30–41 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

