Taspase1 functions as a non-oncogene addiction protease that coordinates cancer cell proliferation and apoptosis
- PMID: 20516119
- PMCID: PMC2909739
- DOI: 10.1158/0008-5472.CAN-10-0027
Taspase1 functions as a non-oncogene addiction protease that coordinates cancer cell proliferation and apoptosis
Abstract
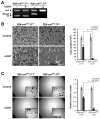
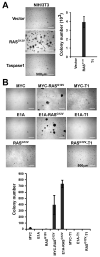
Taspase1, the mixed lineage leukemia and TFIIAalpha-beta cleaving protease, enables cell proliferation and permits oncogenic initiation. Here, we show its critical role in cancer maintenance and thus offer a new anticancer target. Taspase1 is overexpressed in primary human cancers, and deficiency of Taspase1 in cancer cells not only disrupts proliferation but also enhances apoptosis. Mechanistically, loss of Taspase1 induces the levels of CDK inhibitors (CDKI: p16, p21, and p27) and reduces the level of antiapoptotic MCL-1. Therapeutically, deficiency of Taspase1 synergizes with chemotherapeutic agents and ABT-737, an inhibitor of BCL-2/BCL-X(L), to kill cancer cells. Taspase1 alone or in conjunction with MYC, RAS, or E1A fails to transform NIH/3T3 cells or primary mouse embryonic fibroblasts, respectively, but plays critical roles in cancer initiation and maintenance. Therefore, Taspase1 is better classified as a "non-oncogene addiction" protease, the inhibition of which may offer a novel anticancer therapeutic strategy. The reliance of oncogenes on subordinate non-oncogenes during tumorigenesis underscores the non-oncogene addiction hypothesis in which a large class of non-oncogenes functions to maintain cancer phenotypes and presents attractive anticancer therapeutic targets. The emergence of successful cancer therapeutics targeting non-oncogenes to which cancers are addicted supports the future development and potential application of small-molecule Taspase1 inhibitors for cancer therapy.
Copyright 2010 AACR.
Figures




References
-
- Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nature medicine. 2004;10:789–99. - PubMed
-
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. - PubMed
-
- Weinstein IB, Joe A. Oncogene addiction. Cancer Res. 2008;68:3077–80. discussion 80. - PubMed
-
- Zhang J, Yang PL, Gray NS. Targeting cancer with small molecule kinase inhibitors. Nat Rev Cancer. 2009;9:28–39. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

