Epidermal growth factor receptor activation remodels the plasma membrane lipid environment to induce nanocluster formation
- PMID: 20516214
- PMCID: PMC2916403
- DOI: 10.1128/MCB.01615-09
Epidermal growth factor receptor activation remodels the plasma membrane lipid environment to induce nanocluster formation
Abstract
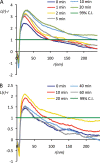
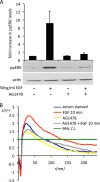
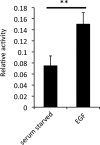
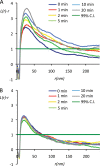
Signal transduction is regulated by the lateral segregation of proteins into nanodomains on the plasma membrane. However, the molecular mechanisms that regulate the lateral segregation of cell surface receptors, such as receptor tyrosine kinases, upon ligand binding are unresolved. Here we used high-resolution spatial mapping to investigate the plasma membrane nanoscale organization of the epidermal growth factor (EGF) receptor (EGFR). Our data demonstrate that in serum-starved cells, the EGFR exists in preformed, cholesterol-dependent, actin-independent nanoclusters. Following stimulation with EGF, the number and size of EGFR nanoclusters increase in a time-dependent manner. Our data show that the formation of EGFR nanoclusters requires receptor tyrosine kinase activity. Critically, we show for the first time that production of phosphatidic acid by phospholipase D2 (PLD2) is essential for ligand-induced EGFR nanocluster formation. In accordance with its crucial role in regulating EGFR nanocluster formation, we demonstrate that modulating PLD2 activity tunes the degree of EGFR nanocluster formation and mitogen-activated protein kinase signal output. Together, these data show that EGFR activation drives the formation of signaling domains by regulating the production of critical second-messenger lipids and modifying the local membrane lipid environment.
Figures



References
-
- Besag, J. E. 1977. Contribution to the discussion of Dr. Ripley's paper. J. R. Statist. Soc. B 39:193-195.
-
- Chen, X., and M. D. Resh. 2002. Cholesterol depletion from the plasma membrane triggers ligand-independent activation of the epidermal growth factor receptor. J. Biol. Chem. 277:49631-49637. - PubMed
-
- Chung, I., R. Akita, R. Vandlen, D. Toomre, J. Schlessinger, and I. Mellman. 2010. Spatial control of EGF receptor activation by reversible dimerization on living cells. Nature 464:783-787. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
