Inhibition of dengue virus by an ester prodrug of an adenosine analog
- PMID: 20516277
- PMCID: PMC2916325
- DOI: 10.1128/AAC.00397-10
Inhibition of dengue virus by an ester prodrug of an adenosine analog
Abstract
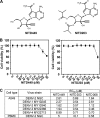
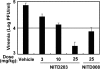
Dengue virus (DENV) is the most prevalent mosquito-borne viral pathogen that infects humans. Neither a vaccine nor an antiviral therapy is currently available for DENV. Here, we report an adenosine nucleoside prodrug that potently inhibits DENV replication both in cell culture and in a DENV mouse model. NITD449 (2'-C-acetylene-7-deaza-7-carbamoyladenosine) was initially identified as a parental compound that inhibits all four serotypes of DENV with low cytotoxicity. However, in vivo pharmacokinetic studies indicated that NITD449 had a low level of exposure in plasma when dosed orally. To increase the oral bioavailability, we covalently linked isobutyric acids to the 3'- and 5'-hydroxyl groups of ribose via ester linkage to NITD449, leading to the prodrug NITD203 (3',5'-O-diisobutyryl-2'-C-acetylene-7-deaza-7-carbamoyl-adenosin). Pharmacokinetic analysis showed that upon oral dosing of the prodrug, NITD203 was readily converted to NITD449, resulting in improved exposure of the parental compound in plasma in both mouse and rat. In DENV-infected AG129 mice, oral dosing of the prodrug at 25 mg/kg of body weight reduced peak viremia by 30-fold. Antiviral spectrum analysis showed that NITD203 inhibited various flaviviruses (DENV, yellow fever virus, and West Nile virus) and hepatitis C virus but not Chikungunya virus (an alphavirus). Mode-of-action analysis, using a luciferase-reporting replicon, indicated that NITD203 inhibited DENV RNA synthesis. Although NITD203 exhibited potent in vitro and in vivo efficacies, the compound could not reach a satisfactory no-observable-adverse-effect level (NOAEL) in a 2-week in vivo toxicity study. Nevertheless, our results demonstrate that a prodrug approach using a nucleoside analog could potentially be developed for flavivirus antiviral therapy.
Figures


Similar articles
-
An adenosine nucleoside inhibitor of dengue virus.Proc Natl Acad Sci U S A. 2009 Dec 1;106(48):20435-9. doi: 10.1073/pnas.0907010106. Epub 2009 Nov 16. Proc Natl Acad Sci U S A. 2009. PMID: 19918064 Free PMC article.
-
Inhibition of dengue virus through suppression of host pyrimidine biosynthesis.J Virol. 2011 Jul;85(13):6548-56. doi: 10.1128/JVI.02510-10. Epub 2011 Apr 20. J Virol. 2011. PMID: 21507975 Free PMC article.
-
Evaluation of AT-752, a Double Prodrug of a Guanosine Nucleotide Analog with In Vitro and In Vivo Activity against Dengue and Other Flaviviruses.Antimicrob Agents Chemother. 2021 Oct 18;65(11):e0098821. doi: 10.1128/AAC.00988-21. Epub 2021 Aug 23. Antimicrob Agents Chemother. 2021. PMID: 34424050 Free PMC article.
-
Ten years of dengue drug discovery: progress and prospects.Antiviral Res. 2013 Nov;100(2):500-19. doi: 10.1016/j.antiviral.2013.09.013. Epub 2013 Sep 27. Antiviral Res. 2013. PMID: 24076358 Review.
-
The search for nucleoside/nucleotide analog inhibitors of dengue virus.Antiviral Res. 2015 Oct;122:12-9. doi: 10.1016/j.antiviral.2015.07.010. Epub 2015 Aug 1. Antiviral Res. 2015. PMID: 26241002 Review.
Cited by
-
Mouse models to study dengue virus immunology and pathogenesis.Front Immunol. 2014 Apr 10;5:151. doi: 10.3389/fimmu.2014.00151. eCollection 2014. Front Immunol. 2014. PMID: 24782859 Free PMC article. Review.
-
Nucleoside inhibitors of tick-borne encephalitis virus.Antimicrob Agents Chemother. 2015 Sep;59(9):5483-93. doi: 10.1128/AAC.00807-15. Epub 2015 Jun 29. Antimicrob Agents Chemother. 2015. PMID: 26124166 Free PMC article.
-
Antiviral Agents in Development for Zika Virus Infections.Pharmaceuticals (Basel). 2019 Jun 29;12(3):101. doi: 10.3390/ph12030101. Pharmaceuticals (Basel). 2019. PMID: 31261947 Free PMC article. Review.
-
Novel and repurposed antiviral molecules for arbovirus infections with epidemic Potential: A systematic review.New Microbes New Infect. 2025 Jul 10;66:101614. doi: 10.1016/j.nmni.2025.101614. eCollection 2025 Aug. New Microbes New Infect. 2025. PMID: 40776987 Free PMC article. Review.
-
Single dose of an adenovirus vectored mouse interferon-α protects mice from lethal EV71 challenge.Antiviral Res. 2016 Oct;134:207-215. doi: 10.1016/j.antiviral.2016.09.003. Epub 2016 Sep 10. Antiviral Res. 2016. PMID: 27623347 Free PMC article.
References
-
- Ali, S., V. Leveque, S. Le Pogam, H. Ma, F. Philipp, N. Inocencio, M. Smith, A. Alker, H. Kang, I. Najera, K. Klumpp, J. Symons, N. Cammack, and W. R. Jiang. 2008. Selected replicon variants with low-level in vitro resistance to the hepatitis C virus NS5B polymerase inhibitor PSI-6130 lack cross-resistance with R1479. Antimicrob. Agents Chemother. 52:4356-4369. - PMC - PubMed
-
- Chen, Y.-L., Z. Yin, J. Duraiswamy, W. Schul, C. C. Lim, B. Liu, H. Y. Xu, M. Qing, A. Yip, G. Wang, W. L. Chan, H. P. Tan, M. Lo, S. Liung, R. R. Kondreddi, R. Rao, H. Gu, H. He, T. H. Keller, and P.-Y. Shi. 2010. Inhibition of dengue virus RNA synthesis by an adenosine nucleoside. Antimicrob. Agents Chemother. 54:2932-2939. - PMC - PubMed
-
- Cote, H. C., Z. L. Brumme, K. J. Craib, C. S. Alexander, B. Wynhoven, L. Ting, H. Wong, M. Harris, P. R. Harrigan, M. V. O'Shaughnessy, and J. S. Montaner. 2002. Changes in mitochondrial DNA as a marker of nucleoside toxicity in HIV-infected patients. N. Engl. J. Med. 346:811-820. - PubMed
-
- De Clercq, E., and J. Neyts. 2009. Antiviral agents acting as DNA or RNA chain terminators. Handb. Exp. Pharmacol. 2009:53-84. - PubMed
-
- Gubler, D., G. Kuno, and L. Markoff. 2007. Flaviviruses, p. 1153-1253. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 5th ed., vol. 1. Lippincott William & Wilkins, Philadelphia, PA.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

