Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates
- PMID: 20516446
- PMCID: PMC4834717
- DOI: 10.1200/JCO.2009.26.7609
Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates
Abstract
Purpose: Programmed death-1 (PD-1), an inhibitory receptor expressed on activated T cells, may suppress antitumor immunity. This phase I study sought to determine the safety and tolerability of anti-PD-1 blockade in patients with treatment-refractory solid tumors and to preliminarily assess antitumor activity, pharmacodynamics, and immunologic correlates.
Patients and methods: Thirty-nine patients with advanced metastatic melanoma, colorectal cancer (CRC), castrate-resistant prostate cancer, non-small-cell lung cancer (NSCLC), or renal cell carcinoma (RCC) received a single intravenous infusion of anti-PD-1 (MDX-1106) in dose-escalating six-patient cohorts at 0.3, 1, 3, or 10 mg/kg, followed by a 15-patient expansion cohort at 10 mg/kg. Patients with evidence of clinical benefit at 3 months were eligible for repeated therapy.
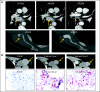
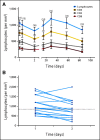
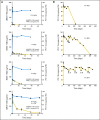
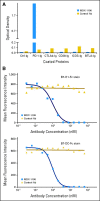
Results: Anti-PD-1 was well tolerated: one serious adverse event, inflammatory colitis, was observed in a patient with melanoma who received five doses at 1 mg/kg. One durable complete response (CRC) and two partial responses (PRs; melanoma, RCC) were seen. Two additional patients (melanoma, NSCLC) had significant lesional tumor regressions not meeting PR criteria. The serum half-life of anti-PD-1 was 12 to 20 days. However, pharmacodynamics indicated a sustained mean occupancy of > 70% of PD-1 molecules on circulating T cells > or = 2 months following infusion, regardless of dose. In nine patients examined, tumor cell surface B7-H1 expression appeared to correlate with the likelihood of response to treatment.
Conclusion: Blocking the PD-1 immune checkpoint with intermittent antibody dosing is well tolerated and associated with evidence of antitumor activity. Exploration of alternative dosing regimens and combinatorial therapies with vaccines, targeted therapies, and/or other checkpoint inhibitors is warranted.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures


Republished in
-
Phase I Study of Single-Agent Anti-Programmed Death-1 (MDX-1106) in Refractory Solid Tumors: Safety, Clinical Activity, Pharmacodynamics, and Immunologic Correlates.J Clin Oncol. 2023 Feb 1;41(4):715-723. doi: 10.1200/JCO.22.02270. J Clin Oncol. 2023. PMID: 36706735 Clinical Trial.
Comment in
-
Immunotherapy to overcome lung tumor cell-induced escape from immunosurveillance.Immunotherapy. 2010 Nov;2(6):757-60. doi: 10.2217/imt.10.62. Immunotherapy. 2010. PMID: 21091107 No abstract available.
References
-
- Segal NH, Parsons DW, Peggs KS, et al. Epitope landscape in breast and colorectal cancer. Cancer Res. 2008;68:889–892. - PubMed
-
- Smyth MJ, Dunn GP, Schreiber RD. Cancer immunosurveillance and immunoediting: The roles of immunity in suppressing tumor development and shaping tumor immunogenicity. Adv Immunol. 2006;90:1–50. - PubMed
-
- Topalian SL, Chen L, Taube J, et al. Immunology. In: Balch C, Houghton AN, Sober AJ, et al., editors. Cutaneous Melanoma. ed 5. St. Louis, MO: Quality Medical Publishing; 2009. pp. 865–882.
-
- Waterhouse P, Penninger JM, Timms E, et al. Lymphoproliferative disorders with early lethality in mice deficient in CTLA-4. Science. 1995;270:985–988. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

