Transient ATM kinase inhibition disrupts DNA damage-induced sister chromatid exchange
- PMID: 20516478
- PMCID: PMC3261617
- DOI: 10.1126/scisignal.2000758
Transient ATM kinase inhibition disrupts DNA damage-induced sister chromatid exchange
Abstract
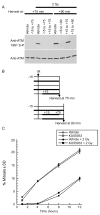
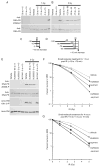
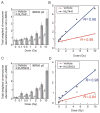
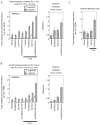
Cells derived from ataxia telangiectasia (A-T) patients exhibit defective cell cycle checkpoints because of mutations in the gene encoding ATM (ataxia telangiectasia mutated). After exposure to ionizing radiation (IR), A-T cells exhibit sensitivity to IR-induced cellular damage that results in increased chromosome aberrations and cell death (radiosensitivity). ATM is a member of a family of kinases that become activated in response to DNA damage. We showed that even transient inhibition of ATM kinase for 1 hour, initiated 15 minutes after cellular irradiation, resulted in an accumulation of persistent chromosome aberrations and increased cell death. Using reversible inhibitors of DNA-PK (DNA-dependent protein kinase), another kinase involved in responding to DNA damage, and ATM, we showed that these two kinases acted through distinct DNA repair mechanisms: ATM resolved DNA damage through a mechanism involving sister chromatid exchange (SCE), whereas DNA-PK acted through nonhomologous end joining. Furthermore, because DNA damage-induced SCE occurred in A-T fibroblasts that lack functional ATM protein, and the inhibitors of ATM kinase had no effect on DNA damage-induced SCE in A-T fibroblasts, we showed that the consequences of short-term inhibition of the kinase activity of ATM and adaptation to ATM protein disruption were distinct. This suggests that A-T fibroblasts have adapted to the loss of ATM and have alternative mechanisms to initiate SCE.
Figures


Similar articles
-
Inhibition of ATM kinase activity does not phenocopy ATM protein disruption: implications for the clinical utility of ATM kinase inhibitors.Cell Cycle. 2010 Oct 15;9(20):4052-7. doi: 10.4161/cc.9.20.13471. Epub 2010 Oct 27. Cell Cycle. 2010. PMID: 20953138 Free PMC article.
-
Transient inhibition of ATM kinase is sufficient to enhance cellular sensitivity to ionizing radiation.Cancer Res. 2008 Sep 15;68(18):7466-74. doi: 10.1158/0008-5472.CAN-08-0763. Cancer Res. 2008. PMID: 18794134 Free PMC article.
-
Characterization of an NBS1 C-terminal peptide that can inhibit ataxia telangiectasia mutated (ATM)-mediated DNA damage responses and enhance radiosensitivity.Mol Pharmacol. 2007 Aug;72(2):320-6. doi: 10.1124/mol.107.036681. Epub 2007 May 16. Mol Pharmacol. 2007. PMID: 17507690
-
Involvement of DNA-PK and ATM in radiation- and heat-induced DNA damage recognition and apoptotic cell death.J Radiat Res. 2010;51(5):493-501. doi: 10.1269/jrr.10039. Epub 2010 Aug 28. J Radiat Res. 2010. PMID: 20814172 Review.
-
ATM: the protein encoded by the gene mutated in the radiosensitive syndrome ataxia-telangiectasia.Int J Radiat Biol. 1999 Oct;75(10):1201-14. doi: 10.1080/095530099139359. Int J Radiat Biol. 1999. PMID: 10549596 Review.
Cited by
-
ATM kinase inhibition preferentially sensitizes p53-mutant glioma to ionizing radiation.Clin Cancer Res. 2013 Jun 15;19(12):3189-200. doi: 10.1158/1078-0432.CCR-12-3408. Epub 2013 Apr 25. Clin Cancer Res. 2013. PMID: 23620409 Free PMC article.
-
Homologous chromosomes make contact at the sites of double-strand breaks in genes in somatic G0/G1-phase human cells.Proc Natl Acad Sci U S A. 2012 Jun 12;109(24):9454-9. doi: 10.1073/pnas.1205759109. Epub 2012 May 29. Proc Natl Acad Sci U S A. 2012. PMID: 22645362 Free PMC article.
-
ATR kinase activation in G1 phase facilitates the repair of ionizing radiation-induced DNA damage.Nucleic Acids Res. 2013 Dec;41(22):10334-44. doi: 10.1093/nar/gkt833. Epub 2013 Sep 14. Nucleic Acids Res. 2013. PMID: 24038466 Free PMC article.
-
Differential phosphorylation of DNA-PKcs regulates the interplay between end-processing and end-ligation during nonhomologous end-joining.Mol Cell. 2015 Apr 2;58(1):172-85. doi: 10.1016/j.molcel.2015.02.024. Epub 2015 Mar 26. Mol Cell. 2015. PMID: 25818648 Free PMC article.
-
ATM loss leads to synthetic lethality in BRCA1 BRCT mutant mice associated with exacerbated defects in homology-directed repair.Proc Natl Acad Sci U S A. 2017 Jul 18;114(29):7665-7670. doi: 10.1073/pnas.1706392114. Epub 2017 Jun 28. Proc Natl Acad Sci U S A. 2017. PMID: 28659469 Free PMC article.
References
-
- Savitsky K, Bar-Shira A, Gilad S, Rotman G, Ziv Y, Vanagaite L, Tagle DA, Smith S, Uziel T, Sfez S, Ashkenazi M, Pecker I, Frydman M, Harnik R, Patanjali SR, Simmons A, Clines GA, Sartiel A, Gatti RA, Chessa L, Sanal O, Lavin MF, Jaspers NG, Taylor AM, Arlett CF, Miki T, Weissman SM, Lovett M, Collins FS, Shiloh Y. A single ataxia telangiectasia gene with a product similar to PI-3 kinase. Science. 1995;268:1749–1753. - PubMed
-
- Gilad S, Khosravi R, Shkedy D, Uziel T, Ziv Y, Savitsky K, Rotman G, Smith S, Chessa L, Jorgensen TJ, Harnik R, Frydman M, Sanal O, Portnoi S, Goldwicz Z, Jaspers NG, Gatti RA, Lenoir G, Lavin MF, Tatsumi K, Wegner RD, Shiloh Y, Bar-Shira A. Predominance of null mutations in ataxia-telangiectasia. Hum Mol Genet. 1996;5:433–439. - PubMed
-
- Banin S, Moyal L, Shieh S, Taya Y, Anderson CW, Chessa L, Smorodinsky NI, Prives C, Reiss Y, Shiloh Y, Ziv Y. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science. 1998;281:1674–1677. - PubMed
-
- Canman CE, Lim DS, Cimprich KA, Taya Y, Tamai K, Sakaguchi K, Appella E, Kastan MB, Siliciano JD. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science. 1998;281:1677–1679. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

