Comparative analysis of amino acids and amino-acid derivatives in protein crystallization
- PMID: 20516615
- PMCID: PMC2882785
- DOI: 10.1107/S1744309110013710
Comparative analysis of amino acids and amino-acid derivatives in protein crystallization
Abstract
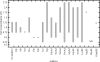
Optimal conditions for protein crystallization are difficult to determine because proteins tend to aggregate in saturated solutions. This study comprehensively evaluates amino acids and amino-acid derivatives as additives for crystallization. This fourth component of the solution increases the probability of crystallization of hen egg-white lysozyme in various precipitants owing to a decrease in aggregation. These results suggest that the addition of certain types of amino acids and amino-acid derivatives, such as Arg, Lys and esterified and amidated amino acids, is a simple method of improving the success rate of protein crystallization.
Figures



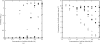

Similar articles
-
Amino acids and glycine ethyl ester as new crystallization reagents for lysozyme.Acta Crystallogr Sect F Struct Biol Cryst Commun. 2010 Jun 1;66(Pt 6):750-4. doi: 10.1107/S174430911001376X. Epub 2010 May 27. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2010. PMID: 20516616 Free PMC article.
-
Amidated amino acids are prominent additives for preventing heat-induced aggregation of lysozyme.J Biosci Bioeng. 2007 May;103(5):440-3. doi: 10.1263/jbb.103.440. J Biosci Bioeng. 2007. PMID: 17609159
-
Control of nucleation in the crystallization of lysozyme.Protein Sci. 1993 Jan;2(1):113-8. doi: 10.1002/pro.5560020112. Protein Sci. 1993. PMID: 8443584 Free PMC article.
-
Dissolution rate of hen egg-white lysozyme crystal under microgravity.Biol Sci Space. 2001 Oct;15 Suppl:S176. doi: 10.2187/bss.15.s176. Biol Sci Space. 2001. PMID: 11799256
-
The effect of hydrolysis time on amino acid analysis.J AOAC Int. 2005 May-Jun;88(3):888-93. J AOAC Int. 2005. PMID: 16001867 Review.
Cited by
-
Thermal aggregation of hen egg white proteins in the presence of salts.Protein J. 2015 Jun;34(3):212-9. doi: 10.1007/s10930-015-9612-3. Protein J. 2015. PMID: 25998040 Free PMC article.
-
Glutathione ethylester, a novel protein refolding reagent, enhances both the efficiency of refolding and correct disulfide formation.Protein J. 2012 Aug;31(6):499-503. doi: 10.1007/s10930-012-9427-4. Protein J. 2012. PMID: 22752753
-
Amino acids and glycine ethyl ester as new crystallization reagents for lysozyme.Acta Crystallogr Sect F Struct Biol Cryst Commun. 2010 Jun 1;66(Pt 6):750-4. doi: 10.1107/S174430911001376X. Epub 2010 May 27. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2010. PMID: 20516616 Free PMC article.
References
-
- Adachi, H., Takano, K., Hosokawa, Y., Inoue, T., Mori, Y., Matsumura, H., Yoshimura, M., Tsunaka, Y., Morikawa, M., Kanaya, S., Masuhara, H., Kai, Y. & Sasaki, T. (2003). Jpn J. Appl. Phys.42, L798–L800.
-
- Adachi, H., Takano, K., Yoshimura, M., Mori, Y. & Sasaki, T. (2002). Jpn J. Appl. Phys.41, L1025–L1027.
-
- Adachi, H., Takano, K., Yoshimura, M., Mori, Y. & Sasaki, T. (2003). Jpn J. Appl. Phys.42, L384–L385.
-
- Arakawa, T. & Tsumoto, K. (2003). Biochem. Biophys. Res. Commun.304, 148–152. - PubMed
-
- Buchner, J. & Rudolph, R. (1991). Biotechnology, 9, 157–162. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

