Methodological quality of preclinical stroke studies is not required for publication in high-impact journals
- PMID: 20517323
- PMCID: PMC2949256
- DOI: 10.1038/jcbfm.2010.74
Methodological quality of preclinical stroke studies is not required for publication in high-impact journals
Abstract
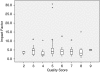
Omitting quality characteristics in animal stroke studies leads to an overestimation of the efficacy of candidate stroke drugs. Nevertheless, the methodological quality of preclinical stroke studies is often limited. As publishing of research results in high-impact journals is an important motivation for scientists, we analyzed whether study quality predicts high-impact publishing. Animal stroke studies of neuroprotective drugs that were recently investigated in clinical phase II/III trials were included in the analysis. Data on the study quality and other important study characteristics were extracted. Regression analyses were performed to estimate the effect of the study characteristics on the journal's impact factor. We identified 117 studies that investigated 12 different drugs. Study quality was not associated with the impact factor before (beta=-0.2, P=0.50) and after adjustment for other study characteristics (beta=-0.3, P=0.19). There was a significant association of the number of investigated mechanisms and applied techniques with the impact factor (beta=1.4, P<0.0001). Our findings show that the quality of animal experimental stroke studies is not relevant for publishing in high-impact journals. The major predictor for accepting preclinical stroke studies in high-impact journals is the complexity of the investigation into a stroke drug's mode of action.
Figures

References
-
- STAIR, Stroke Therapy Academic Industry Roundtable (Fisher M, Chair) Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke. 1999;30:2752–2758. - PubMed
-
- Institute for Scientific Information 2009Web of KnowledgeAvailable at http://www.thompson.isi.com
-
- Adam D. The counting house. Nature. 2002;415:726–729. - PubMed
-
- Avram MJ. Evaluating clinical trials in anesthesia. Anesthesiology. 2002;97:1032–1033. - PubMed
-
- Avram MJ, Shanks CA, Dykes MH, Ronai AK, Stiers WM. Statistical methods in anesthesia articles: an evaluation of two American journals during two six-month periods. Anesth Analg. 1985;64:607–611. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

