The role of feedback in visual masking and visual processing
- PMID: 20517504
- PMCID: PMC2864985
- DOI: 10.2478/v10053-008-0020-5
The role of feedback in visual masking and visual processing
Abstract
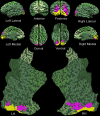
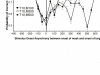
This paper reviews the potential role of feedback in visual masking, for and against. Our analysis reveals constraints for feedback mecha- nisms that limit their potential role in visual masking, and in all other general brain functions. We propose a feedforward model of visual masking, and provide a hypothesis to explain the role of feedback in visual masking and visual processing in general. We review the anato-my and physiology of feedback mechanisms, and propose that the massive ratio of feedback versus feedforward connections in the visual system may be explained solely by the critical need for top-down attentional modulation. We discuss the merits of visual masking as a tool to discover the neural correlates of consciousness, especially as compared to other popular illusions, such as binocular rivalry. Finally, we propose a new set of neurophysiological standards needed to establish whether any given neuron or brain circuit may be the neural substrate of awareness.
Keywords: attention; awareness; consciousness; electrophysiology; fMRI; feedback; humans; masking; metacontrast; monkeys; optical imaging; paracontrast; psychophysics; standing wave; vision; visual.
Figures






Similar articles
-
Dichoptic visual masking reveals that early binocular neurons exhibit weak interocular suppression: implications for binocular vision and visual awareness.J Cogn Neurosci. 2004 Jul-Aug;16(6):1049-59. doi: 10.1162/0898929041502788. J Cogn Neurosci. 2004. PMID: 15298791
-
Paracontrast and metacontrast in schizophrenia: clarifying the mechanism for visual masking deficits.Schizophr Res. 2004 Dec 1;71(2-3):485-92. doi: 10.1016/j.schres.2004.02.018. Schizophr Res. 2004. PMID: 15474919
-
Psychophysical "blinding" methods reveal a functional hierarchy of unconscious visual processing.Conscious Cogn. 2015 Sep;35:234-50. doi: 10.1016/j.concog.2015.01.012. Epub 2015 Feb 18. Conscious Cogn. 2015. PMID: 25704454
-
Probing feedforward and feedback contributions to awareness with visual masking and transcranial magnetic stimulation.Front Psychol. 2014 Oct 21;5:1173. doi: 10.3389/fpsyg.2014.01173. eCollection 2014. Front Psychol. 2014. PMID: 25374548 Free PMC article. Review.
-
Visual masking approaches to visual awareness.Prog Brain Res. 2006;155:177-215. doi: 10.1016/S0079-6123(06)55011-3. Prog Brain Res. 2006. PMID: 17027388 Review.
Cited by
-
Masking of figure-ground texture and single targets by surround inhibition: a computational spiking model.PLoS One. 2012;7(2):e31773. doi: 10.1371/journal.pone.0031773. Epub 2012 Feb 29. PLoS One. 2012. PMID: 22393370 Free PMC article.
-
Does V1 response suppression initiate binocular rivalry?iScience. 2023 Jul 8;26(8):107359. doi: 10.1016/j.isci.2023.107359. eCollection 2023 Aug 18. iScience. 2023. PMID: 37520732 Free PMC article.
-
Human-like perceptual masking is difficult to observe in rats performing an orientation discrimination task.PLoS One. 2018 Nov 21;13(11):e0207179. doi: 10.1371/journal.pone.0207179. eCollection 2018. PLoS One. 2018. PMID: 30462681 Free PMC article.
-
Masking reduces orientation selectivity in rat visual cortex.J Neurophysiol. 2016 Nov 1;116(5):2331-2341. doi: 10.1152/jn.00366.2016. Epub 2016 Aug 17. J Neurophysiol. 2016. PMID: 27535373 Free PMC article.
-
Color and luminance influence, but can not explain, binocular rivalry onset bias.PLoS One. 2011 May 4;6(5):e18978. doi: 10.1371/journal.pone.0018978. PLoS One. 2011. PMID: 21573220 Free PMC article. Clinical Trial.
References
-
- Albrecht D. G., Hamilton D. B. Striate cortex of monkey and cat: contrast response function. Journal of Neurophysiology. 1982;48:217–237. - PubMed
-
- Allman J., Miezin F., McGuinness E. Stimulus specific responses from beyond the classical receptive field: Neurophysiological mechanisms for local-global comparisons in visual neurons. Annual Review of Neuroscience. 1985;8:407–430. - PubMed
-
- Angelucci A., Bressloff P. C. Contribution of feedforward, lateral and feedback connections to the classical receptive field center and extra-classical receptive field surround of primate V1 neurons. Progress in Brain Research. 2006;154:93–120. - PubMed
-
- Angelucci A., Levitt J. B., Lund J. S. Anatomical origins of the classical receptive field and modulatory surround field of single neurons in macaque visual cortical area V1. Progress in Brain Research. 2002;136:373–388. - PubMed
LinkOut - more resources
Full Text Sources
