Synthesis, characterization and reactivity of carbohydrate platinum(IV) complexes with thioglycoside ligands
- PMID: 20517543
- PMCID: PMC3149867
- DOI: 10.1039/b927058b
Synthesis, characterization and reactivity of carbohydrate platinum(IV) complexes with thioglycoside ligands
Abstract
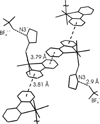
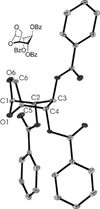
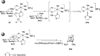
Reactions of fac-[PtMe3(4,4'-R2bpy)(Me2CO)][BF4] (R = H, 1a; tBu, 1b) and fac-[PtMe3(OAc-kappa2O,O')(Me2CO)] (2), respectively, with thioglycosides containing thioethyl (ch-SEt) and thioimidate (ch-STaz, Taz = thiazoline-2-yl) anomeric groups led to the formation of the carbohydrate platinum(IV) complexes fac-[PtMe3(4,4-R2bpy)(ch*)][BF4] (ch* = ch-SEt, 8-14; ch-STaz, 15-23) and fac-[PtMe3(OAc-kappa2O,O')(ch*)] (ch* = ch-SEt, 24-28; ch-STaz = 29-35), respectively. NMR (1H, 13C, 195Pt) spectroscopic investigations and a single-crystal X-ray diffraction analysis of 19 (ch-STaz = 2-thiazolinyl 2,3,4,6-tetra-O-benzoyl-1-thio-beta-D-galactopyranose) revealed the S coordination of the ch-SEt glycosides and the N coordination of the ch-STaz glycosides. Furthermore, X-ray structure analyses of the two decomposition products fac-[PtMe3(bpy)(STazH-kappaS)][BF4] (21a) and 1,6-anhydro-2,3,4-tri-O-benzoyl-beta-D-glucopyranose (23a), where a cleavage of the anomeric C-S bond had occurred in both cases, gave rise to the assumption that this decomposition was mediated due to coordination of the thioglycosides to the high electrophilic platinum(IV) atom, in non-strictly dried solutions. Reactions of fac-[PtMe3(Me2CO)3][BF4] (3) with ch-SEt as well as with ch-SPT and ch-Sbpy thioglycosides (PT = 4-(pyridine-2-yl)-thiazole-2-yl; bpy = 2,2'-bipyridine-6-yl), having N,S and N,N heteroaryl anomeric groups, respectively, led to the formation of platinum(IV) complexes of the type fac-[PtMe3(ch*)][BF4] (ch* = ch-SEt, 36-40, ch-SPT 42-44, ch-Sbpy 45, 46). The thioglycosides were found to be coordinated in a tridentate kappaS,kappa2O,O, kappaS,kappaN,kappaO and kappaS,kappa2N,N coordination mode, respectively. Analogous reactions with ch-STaz ligands succeeded for 2-thiazolinyl 2,3,4-tri-O-benzyl-6-O-(2,2'-bipyridine-6-yl)-1-thio-beta-D-glucopyranoside (5h) resulting in fac-[PtMe3(ch-STaz)][BF4] (41, ch-STaz = 5h), having a kappa3N,N',N''coordinated thioglycoside ligand.
Figures






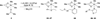

References
-
- Dwek RA. Chem. Rev. 1996;96:683. - PubMed
-
- Lindhorst T. Essentials of Carbohydrate Chemistry. 2nd edn. Weinheim: Wiley-VCH; 2003. p. 175.
-
- Storr T, Merkel M, Song-Zhao GX, Scott LE, Green DE, Bowen ML, Thompson KH, Patrick BO, Schugar HJ, Orvig C. J. Am. Chem. Soc. 2007;129:7453. - PubMed
-
- Hoffman DJ, Whistler RL. Biochemistry. 1968;7:4479. - PubMed
- Whistler RL, Lake WC. Biochem. J. 1972;130:919. - PMC - PubMed
- Hellman B, Lernmark Å, Sehlin J, Täljedal I-B, Whistler RL. Biochem. Pharmacol. 1973;22:29. - PubMed
- Zysk JR, Bushway AA, Whistler RL, Carlton WW. J. Reprod. Fertil. 1975;45:69. - PubMed
-
- Kim JH, Kim SH, Hahn EW, Song CW. Science. 1978;200:206. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

