Cancer inflammation and regulatory T cells
- PMID: 20518013
- PMCID: PMC4068033
- DOI: 10.1002/ijc.25430
Cancer inflammation and regulatory T cells
Abstract
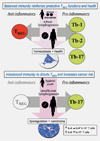
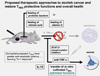
Chronic inflammation is essential for cancer growth and metastasis. It follows that factors reducing inflammation would abrogate cancer and restore tissue health. However, roles for anti-inflammatory CD4+ regulatory cells (T(REG)) in cancer are enigmatic and controversial. Our recent data reveal that T(REG) may function in cancer similarly to inflammatory bowel disease or multiple sclerosis, whereby T(REG) accumulate but lack potency to restore tissue homeostasis under inflammatory conditions. Interestingly, early life exposures to diverse environmental organisms reinforce a protective T(REG) phenotype that inhibits cancer. In contrast, hygienic individuals with few exposures earlier in life suffer from a dysregulated T(REG) feedback loop. Consequently, hygienic subjects have increased risk of malignancy later in life. This cancer condition is reversible by blocking underlying inflammation. Taken together, these data help explain increased inflammation-associated cancer rates in hygienic societies and identify targets to abrogate cancer and restore overall health.
Figures




References
-
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. - PubMed
-
- Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211–217. - PubMed
-
- Balkwill F, Coussens LM. Cancer: an inflammatory link. Nature. 2004;431:405–406. - PubMed
-
- Allavena P, Garlanda C, Borrello MG, Sica A, Mantovani A. Pathways connecting inflammation and cancer. Curr Opin Genet Dev. 2008;18:3–10. - PubMed
-
- Fox JG, Beck P, Dangler CA, Whary MT, Wang TC, Shi HN, Nagler-Anderson C. Concurrent enteric helminth infection modulates inflammation and gastric immune responses and reduces helicobacter-induced gastric atrophy. Nat Med. 2000;6:536–542. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

