Systematic review of antibiograms: A National Laboratory System approach for improving antimicrobial susceptibility testing practices in Michigan
- PMID: 20518446
- PMCID: PMC2846804
- DOI: 10.1177/00333549101250S208
Systematic review of antibiograms: A National Laboratory System approach for improving antimicrobial susceptibility testing practices in Michigan
Abstract
Objectives: Public health surveillance is often dependent on sentinel testing performed in clinical microbiology laboratories, and recognition of emerging/ unusual antimicrobial resistance is especially challenging. We obtained cumulative antibiograms from hospitals to determine whether clinical laboratories recognized unusual resistance or reported antimicrobials inappropriate for various bacterial species, as measured before and after public health laboratory (PHL) educational and technical-support interventions.
Methods: We compared cumulative antibiogram data from 81 clinical laboratories servicing 86 hospitals in Michigan from 2000 through 2005 with a standardized checklist derived from Clinical and Laboratory Standards Institute (CLSI) antimicrobial susceptibility testing (AST) documents. We considered the reporting of unlikely percent-susceptible results and/or inappropriate antimicrobials serious errors, and we calculated error rates for each data year. We used CLSI-recommendation compliance as a measure to determine whether laboratories were implementing changes.
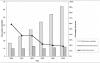
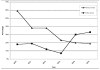
Results: Ninety-five of 239 (28%) cumulative antibiograms examined had one or more serious errors. The annual number of cumulative antibiograms with serious errors did not change radically (range: 10-13); however, when expressed as a percentage of cumulative antibiograms received, the occurrence of these errors declined from 59% in 2000 to 19% in 2005. The reporting of misleading or dangerous antimicrobial-organism combinations occurred less frequently than the reporting of unlikely percent-susceptible results. Compliance with new CLSI recommendations did not improve significantly.
Conclusions: AST is complex and nuanced. PHL programs can provide resources, guidance, and technical support to help clinical microbiologists differentiate questionable AST results from true emerging antimicrobial resistance.
Figures

Similar articles
-
Challenges in Preparation of Cumulative Antibiogram Reports for Community Hospitals.J Clin Microbiol. 2015 Sep;53(9):2977-82. doi: 10.1128/JCM.01077-15. Epub 2015 Jul 15. J Clin Microbiol. 2015. PMID: 26179303 Free PMC article.
-
Antibiogram compliance in University HealthSystem Consortium participating hospitals with Clinical and Laboratory Standards Institute guidelines.Am J Health Syst Pharm. 2012 Apr 1;69(7):598-606. doi: 10.2146/ajhp110332. Am J Health Syst Pharm. 2012. PMID: 22441793
-
Analysis and presentation of cumulative antibiograms: a new consensus guideline from the Clinical and Laboratory Standards Institute.Clin Infect Dis. 2007 Mar 15;44(6):867-73. doi: 10.1086/511864. Epub 2007 Feb 8. Clin Infect Dis. 2007. PMID: 17304462 Review.
-
Systems approach to improving antimicrobial susceptibility testing in clinical laboratories in the United States.J Clin Microbiol. 2007 Jul;45(7):2230-4. doi: 10.1128/JCM.00184-07. Epub 2007 May 23. J Clin Microbiol. 2007. PMID: 17522281 Free PMC article.
-
What's New in Antibiograms? Updating CLSI M39 Guidance with Current Trends.J Clin Microbiol. 2022 Oct 19;60(10):e0221021. doi: 10.1128/jcm.02210-21. Epub 2022 Aug 2. J Clin Microbiol. 2022. PMID: 35916520 Free PMC article. Review.
Cited by
-
Strengthening Public Health in Wisconsin Through the Wisconsin Clinical Laboratory Network.Public Health Rep. 2019 Nov/Dec;134(2_suppl):6S-10S. doi: 10.1177/0033354919837196. Public Health Rep. 2019. PMID: 31682556 Free PMC article.
-
Curbing antimicrobial resistance: Do physicians receive adequate education about antibiograms?J Infect. 2016 Jan;72(1):127-9. doi: 10.1016/j.jinf.2015.09.036. Epub 2015 Oct 9. J Infect. 2016. PMID: 26449880 Free PMC article. No abstract available.
-
The evolving Public Health Laboratory System.Public Health Rep. 2010 May-Jun;125 Suppl 2(Suppl 2):1-3. doi: 10.1177/00333549101250S201. Public Health Rep. 2010. PMID: 20521373 Free PMC article. No abstract available.
-
Antimicrobial Stewardship: How the Microbiology Laboratory Can Right the Ship.Clin Microbiol Rev. 2016 Dec 14;30(1):381-407. doi: 10.1128/CMR.00066-16. Print 2017 Jan. Clin Microbiol Rev. 2016. PMID: 27974411 Free PMC article. Review.
-
Origins and development of the National Laboratory System for public health testing.Public Health Rep. 2010 May-Jun;125 Suppl 2(Suppl 2):18-30. doi: 10.1177/00333549101250S203. Public Health Rep. 2010. PMID: 20518442 Free PMC article.
References
-
- Staphylococcus aureus resistant to vancomycin—United States, 2002. [cited 2009 Mar 14];MMWR Morb Mortal Wkly Rep. 2002 51(26):565–7. Also available from: URL: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5126a1.htm. - PubMed
-
- State of Michigan Office of Administrative Hearings and Rules, Department of Community Health, Bureau of Epidemiology. Communicable disease rules. [cited 2009 Dec 16]. Available from: URL: http://www.state.mi.us/orr/emi/admincode.asp?AdminCode=Single&Admin_Num=....
-
- National Committee for Clinical Laboratory Standards. Analysis and presentation of cumulative antimicrobial susceptibility test data; approved guideline. NCCLS document M39-A. Wayne (PA): NCCLS; 2002.
-
- National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial susceptibility testing; 12th informational supplement. NCCLS document M100-S12. Wayne (PA): NCCLS; 2002.
-
- National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial disk susceptibility tests; approved standard. 8th ed. NCCLS document M2-A8. Wayne (PA): NCCLS; 2003.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

