Implementing routine testing for severe combined immunodeficiency within Wisconsin's newborn screening program
- PMID: 20518449
- PMCID: PMC2846807
- DOI: 10.1177/00333549101250S211
Implementing routine testing for severe combined immunodeficiency within Wisconsin's newborn screening program
Abstract
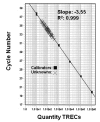
Severe combined immunodeficiency (SCID) is the result of genetic defects that impair normal T-cell development. SCID babies typically appear normal at birth, but acquire multiple life-threatening infections within a few months. Early diagnosis and treatment with a bone-marrow transplant markedly improves long-term outcomes. On January 1, 2008, the newborn screening (NBS) program in Wisconsin became the first in the world to routinely test all newborns for SCID. A realtime quantitative polymerase chain reaction assay measures T-cell receptor excision circles (TRECs), which are formed during the maturation of normal T-cells. A lack or very low number of TRECs is consistent with T-cell lymphopenia. The development and validation of the TREC assay and the results of the first year of screening have been published. This article describes the process used to add SCID to the NBS panel, the establishment of follow-up capacity, and the integration of SCID screening into routine NBS workflows. The development of this expanded NBS program is described so that other states might benefit from the processes used in Wisconsin.
Figures


References
-
- Baker M, Brokopp C, Hoffman G, Laessig R, Kurtycz D, Grossman W, et al. Newborn screening for severe combined immunodeficiencies (SCID)—a 2008 Wisconsin perspective. Proceedings of the 2008 Newborn Screening and Genetic Testing Symposium; 2008 Nov 3–6; San Antonio. Silver Spring (MD): Association of Public Health Laboratories; 2008.
-
- Buckley RH. Molecular defects in human severe combined immunodeficiency and approaches to immune reconstitution. Annu Rev Immunol. 2004;22:625–55. - PubMed
-
- Puck JM. Neonatal screening for severe combined immunodeficiency. Curr Opin Allergy Clin Immunol. 2007;7:522–7. - PubMed
-
- Wilson JMG, Junger G. Principles and practice of screening for disease. Geneva: World Health Organization; 1968.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

