Structure and properties of a bis-histidyl ligated globin from Caenorhabditis elegans
- PMID: 20518498
- PMCID: PMC2903878
- DOI: 10.1021/bi100710a
Structure and properties of a bis-histidyl ligated globin from Caenorhabditis elegans
Abstract
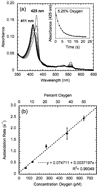
Globins are heme-containing proteins that are best known for their roles in oxygen (O(2)) transport and storage. However, more diverse roles of globins in biology are being revealed, including gas and redox sensing. In the nematode Caenorhabditis elegans, 33 globin or globin-like genes were recently identified, some of which are known to be expressed in the sensory neurons of the worm and linked to O(2) sensing behavior. Here, we describe GLB-6, a novel globin-like protein expressed in the neurons of C. elegans. Recombinantly expressed full-length GLB-6 contains a heme site with spectral features that are similar to those of other bis-histidyl ligated globins, such as neuroglobin and cytoglobin. In contrast to these globins, however, ligands such as CO, NO, and CN(-) do not bind to the heme in GLB-6, demonstrating that the endogenous histidine ligands are likely very tightly coordinated. Additionally, GLB-6 exhibits rapid two-state autoxidation kinetics in the presence of physiological O(2) levels as well as a low redox potential (-193 +/- 2 mV). A high-resolution (1.40 A) crystal structure of the ferric form of the heme domain of GLB-6 confirms both the putative globin fold and bis-histidyl ligation and also demonstrates key structural features that can be correlated with the unusual ligand binding and redox properties exhibited by the full-length protein. Taken together, the biochemical properties of GLB-6 suggest that this neural protein would most likely serve as a physiological sensor for O(2) in C. elegans via redox signaling and/or electron transfer.
Figures




Similar articles
-
Electron transfer function versus oxygen delivery: a comparative study for several hexacoordinated globins across the animal kingdom.PLoS One. 2011;6(6):e20478. doi: 10.1371/journal.pone.0020478. Epub 2011 Jun 1. PLoS One. 2011. PMID: 21674044 Free PMC article.
-
A globin domain in a neuronal transmembrane receptor of Caenorhabditis elegans and Ascaris suum: molecular modeling and functional properties.J Biol Chem. 2015 Apr 17;290(16):10336-52. doi: 10.1074/jbc.M114.576520. Epub 2015 Feb 9. J Biol Chem. 2015. PMID: 25666609 Free PMC article.
-
GLB-3: A resilient, cysteine-rich, membrane-tethered globin expressed in the reproductive and nervous system of Caenorhabditis elegans.J Inorg Biochem. 2023 Jan;238:112063. doi: 10.1016/j.jinorgbio.2022.112063. Epub 2022 Nov 3. J Inorg Biochem. 2023. PMID: 36370505
-
Tracing the structure-function relationship of neuroglobin and cytoglobin using resonance Raman and electron paramagnetic resonance spectroscopy.IUBMB Life. 2004 Nov-Dec;56(11-12):665-70. doi: 10.1080/15216540500037877. IUBMB Life. 2004. PMID: 15804830 Review.
-
Neuroglobin and cytoglobin in search of their role in the vertebrate globin family.J Inorg Biochem. 2005 Jan;99(1):110-9. doi: 10.1016/j.jinorgbio.2004.11.009. J Inorg Biochem. 2005. PMID: 15598495 Review.
Cited by
-
Open and Lys-His Hexacoordinated Closed Structures of a Globin with Swapped Proximal and Distal Sites.Sci Rep. 2015 Jun 22;5:11407. doi: 10.1038/srep11407. Sci Rep. 2015. PMID: 26094577 Free PMC article.
-
Molecular controls of the oxygenation and redox reactions of hemoglobin.Antioxid Redox Signal. 2013 Jun 10;18(17):2298-313. doi: 10.1089/ars.2012.4947. Epub 2013 Jan 21. Antioxid Redox Signal. 2013. PMID: 23198874 Free PMC article. Review.
-
GLOBIN-5-dependent O2 responses are regulated by PDL-1/PrBP that targets prenylated soluble guanylate cyclases to dendritic endings.J Neurosci. 2014 Dec 10;34(50):16726-38. doi: 10.1523/JNEUROSCI.5368-13.2014. J Neurosci. 2014. PMID: 25505325 Free PMC article.
-
Prediction of Protein Function from Tertiary Structure of the Active Site in Heme Proteins by Convolutional Neural Network.Biomolecules. 2023 Jan 9;13(1):137. doi: 10.3390/biom13010137. Biomolecules. 2023. PMID: 36671521 Free PMC article.
-
A redox signalling globin is essential for reproduction in Caenorhabditis elegans.Nat Commun. 2015 Dec 1;6:8782. doi: 10.1038/ncomms9782. Nat Commun. 2015. PMID: 26621324 Free PMC article.
References
-
- de Sanctis D, Dewilde S, Pesce A, Moens L, Ascenzi P, Hankeln T, Burmester T, Bolognesi M. Crystal Structure of cytoglobin: the fourth globin type discovered in man displays heme hexa-coordination. J Mol Biol. 2004;336:917–927. - PubMed
-
- Pesce A, Dewilde S, Nardini M, Moens L, Ascenzi P, Hankeln T, Burmester T, Bolognesi M. Human brain neuroglobin structure reveals a distinct mode of controlling oxygen affinity. Structure. 2003;11:1087–1096. - PubMed
-
- Vallone B, Nienhaus K, Brunori M, Nienhaus GU. The structure of murine neuroglobin: Novel pathways for ligand migration and binding. Proteins. 2004;56:85–92. - PubMed
-
- Dewilde S, Kiger L, Burmester T, Hankeln T, Baudein-Crueza V, Aerts T, Marden MC, Caubergs R, Moens L. Biochemical characterization and ligand binding properties of neuroglobin, a novel member of the globin family. J Biol Chem. 2001;276:38949–38955. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

