Smart lipids for programmable nanomaterials
- PMID: 20518544
- PMCID: PMC2912439
- DOI: 10.1021/nl101640k
Smart lipids for programmable nanomaterials
Abstract
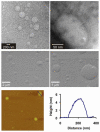
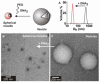
Novel, responsive liposomes are introduced, assembled from DNA-programmed lipids allowing sequence selective manipulation of nanoscale morphology. Short, single-stranded DNA sequences form polar head groups conjugated to hydrophobic tails. The morphology of the resulting lipid aggregates depends on sterics and electronics in the polar head groups and, therefore, is dependent on the DNA hybridization state. The programmability, specificity, and reversibility of the switchable system are demonstrated via dynamic light scattering, transmission electron microscopy, and fluorescence microscopy.
Figures


Similar articles
-
Efficient Fusion of Liposomes by Nucleobase Quadruple-Anchored DNA.Chemistry. 2017 Jul 12;23(39):9391-9396. doi: 10.1002/chem.201701379. Epub 2017 Jun 20. Chemistry. 2017. PMID: 28513997
-
Binding of DNA origami to lipids: maximizing yield and switching via strand displacement.Nucleic Acids Res. 2021 Nov 8;49(19):10835-10850. doi: 10.1093/nar/gkab888. Nucleic Acids Res. 2021. PMID: 34614184 Free PMC article.
-
Hybrid nanocapsules: interactions of ABA block copolymers with liposomes.J Am Chem Soc. 2005 May 4;127(17):6242-7. doi: 10.1021/ja043600x. J Am Chem Soc. 2005. PMID: 15853329
-
Responsive self-assembled nanostructured lipid systems for drug delivery and diagnostics.J Colloid Interface Sci. 2016 Dec 15;484:320-339. doi: 10.1016/j.jcis.2016.08.077. Epub 2016 Sep 1. J Colloid Interface Sci. 2016. PMID: 27623190 Review.
-
Rational design of DNA nanoarchitectures.Angew Chem Int Ed Engl. 2006 Mar 13;45(12):1856-76. doi: 10.1002/anie.200502358. Angew Chem Int Ed Engl. 2006. PMID: 16470892 Review.
Cited by
-
Enhancing the Stability and Immunomodulatory Activity of Liposomal Spherical Nucleic Acids through Lipid-Tail DNA Modifications.Small. 2018 Feb;14(5):10.1002/smll.201702909. doi: 10.1002/smll.201702909. Epub 2017 Dec 11. Small. 2018. PMID: 29226611 Free PMC article.
-
Amphiphilic "Like-a-Brush" Oligonucleotide Conjugates with Three Dodecyl Chains: Self-Assembly Features of Novel Scaffold Compounds for Nucleic Acids Delivery.Nanomaterials (Basel). 2020 Sep 29;10(10):1948. doi: 10.3390/nano10101948. Nanomaterials (Basel). 2020. PMID: 33003636 Free PMC article.
-
Stimuli-responsive nanomaterials for biomedical applications.J Am Chem Soc. 2015 Feb 18;137(6):2140-54. doi: 10.1021/ja510147n. Epub 2015 Feb 6. J Am Chem Soc. 2015. PMID: 25474531 Free PMC article.
-
3D Printed Programmable Release Capsules.Nano Lett. 2015 Aug 12;15(8):5321-9. doi: 10.1021/acs.nanolett.5b01688. Epub 2015 Jun 8. Nano Lett. 2015. PMID: 26042472 Free PMC article.
-
Förster Resonance Energy Transfer Nanoplatform Based on Recognition-Induced Fusion/Fission of DNA Mixed Micelles for Nucleic Acid Sensing.ACS Nano. 2021 May 25;15(5):8517-8524. doi: 10.1021/acsnano.1c00156. Epub 2021 May 7. ACS Nano. 2021. PMID: 33961404 Free PMC article.
References
-
- Gennis RB. Biomembranes. Springer-Verlag; New York: 1989.
-
- Hartgerink JD, Beniash E, Stupp SI. Science. 2001;294:1684–1688. - PubMed
- Discher Dennis E, Eisenberg A. Science. 2002;297:967–73. - PubMed
- Zhang Q, Ariga K, Okabe A, Aida T. J. Am. Chem. Soc. 2004;126:988–989. - PubMed
- Gong Y, Luo Y, Bong D. J. Am. Chem. Soc. 2006;128:14430–14431. - PubMed
- Vriezema DM, Garcia PML, Oltra NS, Natzakis NS, Kuiper SM, Nolte RJM, Rowan AE, van Hest JCM. Angew. Chem., Int. Ed. 2007;46:7378–7382. - PubMed
- Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nat. Nanotechnol. 2007;2:751–760. - PubMed
- Lee S-M, Dettmer CM, O’Halloran TV, Nguyen ST. J. Am. Chem. Soc. 2007;129:15096–15097. - PubMed
- Smart T, Lomas H, Massignani M, Flores-Merino MV, Perez LR, Battaglia G. Nano Today. 2008;3:38–46.
- Chen C-L, Zhang P, Rosi NL. J. Am. Chem. Soc. 2008;130:13555–13557. - PMC - PubMed
- Chandrawati R, Stadler B, Postma A, Connal LA, Chong S-F, Zelikin AN, Caruso F. Biomaterials. 2009;30:5988–5998. - PubMed
- Chan Y-HM, Lengerich B, Boxer SG. Proc. Natl. Acad. Sci. 2009;106:979–984. - PMC - PubMed
-
- Wang Y, Xu H, Zhang X. Adv. Mat. 2009;21:1–16.
-
- Butun V, Billingham NC, Armes SP. J. Am. Chem. Soc. 1998;120:11818–11819.
- Li Y, Du W, Sun G, Wooley KL. Macromolecules. 2008;41:6605–6607.
- Versluis F, Tomatsu I, Kehr S, Fregonese C, Tepper AWJW, Stuart MCA, Ravoo BJ, Koning RI, Kros A. J. Am. Chem. Soc. 2009;131:13186–13187. - PubMed
-
- Sundararaman A, Stephan T, Grubbs RB. J. Am. Chem. Soc. 2008;130:12264–12265. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

