Synthesis and evaluation of amino-modified alpha-GalCer analogues
- PMID: 20518554
- PMCID: PMC2903208
- DOI: 10.1021/ol100934z
Synthesis and evaluation of amino-modified alpha-GalCer analogues
Abstract
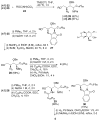
Alpha-GalCer analogues featuring a phytoceramide 3- and 4-amino group have been synthesized. A Mitsunobu reaction involving phthalimide was employed for the introduction of the amino groups at the 3- and 4-positions of suitable phytosphingosine-derived precursors. The influence of these modifications on the interaction with the T-cell receptor of NKT cells was investigated, as well as the capacity of the amino-modified analogues to induce a cytokine response after in vivo administration.
Figures
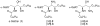
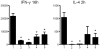
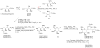

References
-
-
Reviews: Wu D, Fujio M, Wong CH. Bioorg Med Chem. 2008;16:1073.Savage PB, Teyton L, Bendelac A. Chem Soc Rev. 2006;35:771.
-
-
- Morita M, Motoki K, Akimoto K, Natori T, Sakai T, Sawa E, Yamaji K, Koezuka Y, Kobayashi E, Fukushima H. J Med Chem. 1995;38:2176. - PubMed
- Natori T, Koezuka Y, Higa T. Tetrahedron Lett. 1993;34:5591.
- Natori T, Morita M, Akimoto K, Koezuka Y. Tetrahedron. 1994;50:2771.
-
- Berkers CR, Ovaa H. Trends Pharmacol Sci. 2005;26:252. - PubMed
-
- Koch M, Stronge VS, Shepherd D, Gadola SD, Mathew B, Ritter G, Fersht GS, Schmidt RR, Jones EY, Cerundolo V. Nat Immunol. 2005;6:819. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

