Contribution of light chain residues to high affinity binding in an HIV-1 antibody explored by combinatorial scanning mutagenesis
- PMID: 20518570
- PMCID: PMC2911358
- DOI: 10.1021/bi100293q
Contribution of light chain residues to high affinity binding in an HIV-1 antibody explored by combinatorial scanning mutagenesis
Abstract
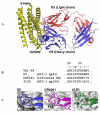
Detailed analysis of factors governing high affinity antibody-antigen interactions yields important insight into molecular recognition and facilitates the design of functional antibody libraries. Here we describe comprehensive mutagenesis of the light chain complementarity determining regions (CDRs) of HIV-1 antibody D5 (which binds its target, "5-Helix", with a reported K(D) of 50 pM). Combinatorial scanning mutagenesis libraries were prepared in which CDR residues on the D5 light chain were varied among WT side chain identity or alanine. Selection of these libraries against 5-Helix and then sequence analysis of the resulting population were used to quantify energetic consequences of mutation from wild-type to alanine (DeltaDeltaG(Ala-WT)) at each position. This analysis revealed several hotspot residues (DeltaDeltaG(Ala-WT) >or= 1 kcal/mol) that formed combining site features critical to the affinity of the interaction. Tolerance of D5 light chain residues to alternative mutations was explored with a second library. We found that light chain residues located at the center and at the periphery of the D5 combining site contribute to shape complementarity and electrostatic characteristics. Thus, the affinity of D5 for 5-Helix arises from extended interactions involving both the heavy and light chains of D5. These results provide significant insight for future antibody engineering efforts.
Figures




Similar articles
-
Side chain requirements for affinity and specificity in D5, an HIV-1 antibody derived from the VH1-69 germline segment.BMC Biochem. 2013 Apr 8;14:9. doi: 10.1186/1471-2091-14-9. BMC Biochem. 2013. PMID: 23566198 Free PMC article.
-
Humanization of a murine monoclonal antibody by simultaneous optimization of framework and CDR residues.J Mol Biol. 1999 Nov 19;294(1):151-62. doi: 10.1006/jmbi.1999.3141. J Mol Biol. 1999. PMID: 10556035
-
CDR walking mutagenesis for the affinity maturation of a potent human anti-HIV-1 antibody into the picomolar range.J Mol Biol. 1995 Dec 1;254(3):392-403. doi: 10.1006/jmbi.1995.0626. J Mol Biol. 1995. PMID: 7490758
-
Comprehensive functional maps of the antigen-binding site of an anti-ErbB2 antibody obtained with shotgun scanning mutagenesis.J Mol Biol. 2002 Jul 5;320(2):415-28. doi: 10.1016/S0022-2836(02)00264-4. J Mol Biol. 2002. PMID: 12079396
-
Affinity maturation and characterization of a human monoclonal antibody against HIV-1 gp41.MAbs. 2009 Sep-Oct;1(5):462-74. doi: 10.4161/mabs.1.5.9214. Epub 2009 Sep 8. MAbs. 2009. PMID: 20065653 Free PMC article.
Cited by
-
Protein and Antibody Engineering by Phage Display.Methods Enzymol. 2016;580:45-87. doi: 10.1016/bs.mie.2016.05.005. Epub 2016 Jun 29. Methods Enzymol. 2016. PMID: 27586328 Free PMC article.
-
Comprehensive mapping of functional epitopes on dengue virus glycoprotein E DIII for binding to broadly neutralizing antibodies 4E11 and 4E5A by phage display.Virology. 2015 Nov;485:371-82. doi: 10.1016/j.virol.2015.08.011. Epub 2015 Sep 2. Virology. 2015. PMID: 26339794 Free PMC article.
-
Role of antibody heavy and light chain interface residues in affinity maturation of binding to HIV envelope glycoprotein.Mol Syst Des Eng. 2019 Aug 2;4(4):737-746. doi: 10.1039/c8me00080h. Epub 2019 Feb 8. Mol Syst Des Eng. 2019. PMID: 40546888 Free PMC article.
-
Side chain requirements for affinity and specificity in D5, an HIV-1 antibody derived from the VH1-69 germline segment.BMC Biochem. 2013 Apr 8;14:9. doi: 10.1186/1471-2091-14-9. BMC Biochem. 2013. PMID: 23566198 Free PMC article.
-
Synthetic antibodies with a human framework that protect mice from lethal Sudan ebolavirus challenge.ACS Chem Biol. 2014 Oct 17;9(10):2263-73. doi: 10.1021/cb5006454. Epub 2014 Aug 26. ACS Chem Biol. 2014. PMID: 25140871 Free PMC article.
References
-
- Maynard J, Georgiou G. Antibody engineering. Annu. Rev. Biomed. Eng. 2000;2:339–376. - PubMed
-
- MacCallum RM, Martin CA, Thornton JM. Antibody-antigen interactions: contact analysis and binding site topography. J. Mol. Biol. 1996;262:732–745. - PubMed
-
- Almagro JC. Identification of differences in the specificity-determining residues of antibodies that recognize antigens of different size: implications for the rational design of antibody repertoires. J. Mol. Recognit. 2004;17:132–143. - PubMed
-
- Wilson IA, Stanfield RL. Antibody-antigen interactions: new structures and conformational changes. Curr. Opin. Struct. Biol. 1994;4:857–867. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

