Insulin signaling and insulin sensitizing in muscle and liver of obese monkeys: peroxisome proliferator-activated receptor gamma agonist improves defective activation of atypical protein kinase C
- PMID: 20518698
- PMCID: PMC3014763
- DOI: 10.1089/ars.2010.3234
Insulin signaling and insulin sensitizing in muscle and liver of obese monkeys: peroxisome proliferator-activated receptor gamma agonist improves defective activation of atypical protein kinase C
Abstract
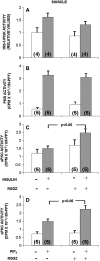
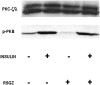
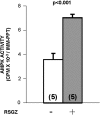
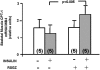
Obesity, the metabolic syndrome, and aging share several pathogenic features in both humans and non-human primates, including insulin resistance and inflammation. Since muscle and liver are considered key integrators of metabolism, we sought to determine in biopsies from lean and obese aging rhesus monkeys the nature of defects in insulin activation and, further, the potential for mitigation of such defects by an in vivo insulin sensitizer, rosiglitazone, and a thiazolidinedione activator of the peroxisome proliferator-activated receptor gamma. The peroxisome proliferator-activated receptor gamma agonist reduced hyperinsulinemia, improved insulin sensitivity, lowered plasma triglycerides and free fatty acids, and increased plasma adiponectin. In muscle of obese monkeys, previously shown to exhibit defective insulin signaling, the insulin sensitizer improved insulin activation of atypical protein kinase C (aPKC), the defective direct activation of aPKC by phosphatidylinositol (PI)-3,4,5-(PO₄)₃, and 5'-AMP-activated protein kinase and increased carnitine palmitoyltransferase-1 mRNA expression, but it did not improve insulin activation of insulin receptor substrate (IRS)-1-dependent PI 3-kinase (IRS-1/PI3K), protein kinase B, or glycogen synthase. We found that, although insulin signaling was impaired in muscle, insulin activation of IRS-1/PI3K, IRS-2/PI3K, protein kinase B, and aPKC was largely intact in liver and that rosiglitazone improved insulin signaling to aPKC in muscle by improving responsiveness to PI-3,4,5-(PO₄)₃.
Figures


References
-
- Bae SS. Cho H. Mu J. Birnbaum MJ. Isoform-specific regulation of insulin-dependent glucose uptake by Akt/protein kinase B. J Biol Chem. 2003;278:49530–49536. - PubMed
-
- Bandyopadhyay G. Kanoh Y. Sajan MP. Standaert ML. Farese RV. Effects of adenoviral gene transfer of wild-type, constitutively active, and kinase-defective protein kinase C-lambda on insulin-stimulated glucose transport in L6 myotubes. Endocrinology. 2000;141:4120–4127. - PubMed
-
- Bandyopadhyay G. Sajan MP. Kanoh Y. Standaert ML. Quon MJ. Lea-Currie R. Sen A. Farese RV. PKC-zeta mediates insulin effects on glucose transport in cultured preadipocyte-derived human adipocytes. J Clin Endocrinol Metab. 2002;87:716–723. - PubMed
-
- Bandyopadhyay G. Standaert ML. Galloway L. Moscat J. Farese RV. Evidence for involvement of protein kinase C (PKC)-zeta and noninvolvement of diacylglycerol-sensitive PKCs in insulin-stimulated glucose transport in L6 myotubes. Endocrinology. 1997;138:4721–4731. - PubMed
-
- Bandyopadhyay G. Standaert ML. Zhao L. Yu B. Avignon A. Galloway L. Karnam P. Moscat J. Farese RV. Activation of protein kinase C (alpha, beta, and zeta) by insulin in 3T3/L1 cells. Transfection studies suggest a role for PKC-zeta in glucose transport. J Biol Chem. 1997;272:2551–2558. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

