Hypothalamic nitric oxide in hypoglycemia detection and counterregulation: a two-edged sword
- PMID: 20518706
- PMCID: PMC3025177
- DOI: 10.1089/ars.2010.3331
Hypothalamic nitric oxide in hypoglycemia detection and counterregulation: a two-edged sword
Abstract
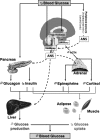
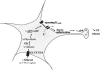
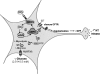
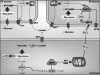
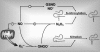
Hypoglycemia is the main complication for patients with type 1 diabetes mellitus receiving intensive insulin therapy. In addition to the obvious deleterious effects of acute hypoglycemia on brain function, recurrent episodes of hypoglycemia (RH) have an even more insidious effect. RH impairs the ability of the brain to detect and initiate an appropriate counterregulatory response (CRR) to restore euglycemia in response to subsequent hypoglycemia. Knowledge of mechanisms involved in hypoglycemia detection and counterregulation has significantly improved over the past 20 years. Glucose sensitive neurons (GSNs) in the ventromedial hypothalamus (VMH) may play a key role in the CRR. VMH nitric oxide (NO) production has recently been shown to be critical for both the CRR and glucose sensing by glucose-inhibited neurons. Interestingly, downstream effects of NO may also contribute to the impaired CRR after RH. In this review, we will discuss current literature regarding the molecular mechanisms by which VMH GSNs sense glucose. Putative roles of GSNs in the detection and initiation of the CRR will then be described. Finally, hypothetical mechanisms by which VMH NO production may both facilitate and subsequently impair the CRR will be discussed.
Figures


References
-
- The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Eng J Med. 1993;329:977–986. - PubMed
-
- Almeida A. Moncada S. Bolanos JP. Nitric oxide switches on glycolysis through the AMP protein kinase and 6-phosphofructo-2-kinase pathway. Nat Cell Biol. 2004;6:45–51. - PubMed
-
- Alquier T. Kawashima J. Tsuji Y. Kahn BB. Role of hypothalamic Amp kinase in the impaired counterregulatory response induced by repetitive neuroglucopenia. Endocrinology. 2006;148:1367–1375. - PubMed
-
- Alquier T. Leloup C. Arnaud E. Magnan C. Penicaud L. Altered Glut4 mRNA levels in specific brain areas of hyperglycemic- hyperinsulinemic rats. Neurosci Lett. 2001;308:75–78. - PubMed
-
- Anand BK. Chhina GS. Sharma KN. Dua S. Singh B. Activity of single neurons in the hypothalamic feeding centers: effect of glucose. Am J Physiol. 1964;207:1146–1154. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

