LAMP-2-deficient human B cells exhibit altered MHC class II presentation of exogenous antigens
- PMID: 20518820
- PMCID: PMC2996552
- DOI: 10.1111/j.1365-2567.2010.03309.x
LAMP-2-deficient human B cells exhibit altered MHC class II presentation of exogenous antigens
Abstract
Major histocompatibility complex (MHC) class II molecules present antigenic peptides derived from engulfed exogenous proteins to CD4(+) T cells. Exogenous antigens are processed in mature endosomes and lysosomes where acidic proteases reside and peptide-binding to class II alleles is favoured. Hence, maintenance of the microenvironment within these organelles is probably central to efficient MHC class II-mediated antigen presentation. Lysosome-associated membrane proteins such as LAMP-2 reside in mature endosomes and lysosomes, yet their role in exogenous antigen presentation pathways remains untested. In this study, human B cells lacking LAMP-2 were examined for changes in MHC class II-restricted antigen presentation. MHC class II presentation of exogenous antigen and peptides to CD4(+) T cells was impaired in the LAMP-2-deficient B cells. Peptide-binding to MHC class II on LAMP-2-deficient B cells was reduced at physiological pH compared with wild-type cells. However, peptide-binding and class II-restricted antigen presentation were restored by incubation of LAMP-2-negative B cells at acidic pH, suggesting that efficient loading of exogenous epitopes by MHC class II molecules is dependent upon LAMP-2 expression in B cells. Interestingly, class II presentation of an epitope derived from an endogenous transmembrane protein was detected using LAMP-2-deficient B cells. Consequently, LAMP-2 may control the repertoire of peptides displayed by MHC class II molecules on B cells and influence the balance between endogenous and exogenous antigen presentation.
© 2010 The Authors. Immunology © 2010 Blackwell Publishing Ltd.
Figures

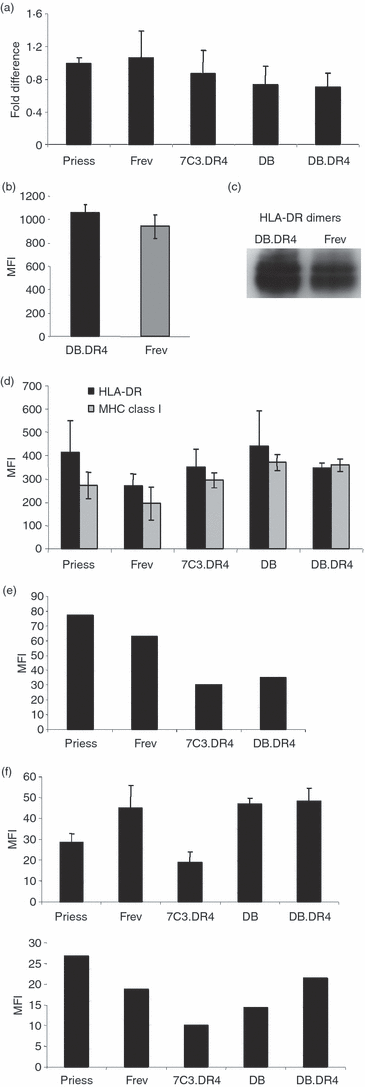
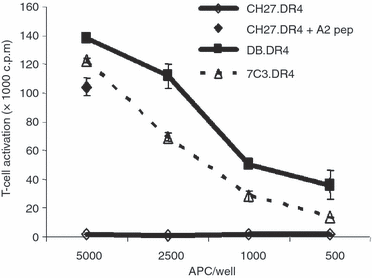
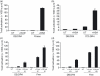
Similar articles
-
Loading of MHC class I and II presentation pathways by exogenous antigens: a quantitative in vivo comparison.J Immunol. 2004 May 15;172(10):6129-35. doi: 10.4049/jimmunol.172.10.6129. J Immunol. 2004. PMID: 15128799
-
Lamp-2a facilitates MHC class II presentation of cytoplasmic antigens.Immunity. 2005 May;22(5):571-81. doi: 10.1016/j.immuni.2005.03.009. Immunity. 2005. PMID: 15894275
-
Presentation of a self-peptide for in vivo tolerance induction of CD4+ T cells is governed by a processing factor that maps to the class II region of the major histocompatibility complex locus.J Exp Med. 1995 Nov 1;182(5):1481-91. doi: 10.1084/jem.182.5.1481. J Exp Med. 1995. PMID: 7595218 Free PMC article.
-
Mechanistic understanding and significance of small peptides interaction with MHC class II molecules for therapeutic applications.Immunol Rev. 2016 Jul;272(1):151-68. doi: 10.1111/imr.12435. Immunol Rev. 2016. PMID: 27319349 Review.
-
Endogenous antigen presentation by MHC class II molecules.Immunol Res. 1994;13(4):253-67. doi: 10.1007/BF02935617. Immunol Res. 1994. PMID: 7616053 Review.
Cited by
-
Melanoma LAMP-2C Modulates Tumor Growth and Autophagy.Front Cell Dev Biol. 2018 Aug 29;6:101. doi: 10.3389/fcell.2018.00101. eCollection 2018. Front Cell Dev Biol. 2018. PMID: 30211163 Free PMC article.
-
Escape from X chromosome inactivation and female bias of autoimmune diseases.Mol Med. 2020 Dec 9;26(1):127. doi: 10.1186/s10020-020-00256-1. Mol Med. 2020. PMID: 33297945 Free PMC article. Review.
-
Orchestrating Resilience: How Neuropilin-2 and Macrophages Contribute to Cardiothoracic Disease.J Clin Med. 2024 Mar 1;13(5):1446. doi: 10.3390/jcm13051446. J Clin Med. 2024. PMID: 38592275 Free PMC article. Review.
-
Cutting edge: NADPH oxidase modulates MHC class II antigen presentation by B cells.J Immunol. 2012 Oct 15;189(8):3800-4. doi: 10.4049/jimmunol.1103080. Epub 2012 Sep 14. J Immunol. 2012. PMID: 22984083 Free PMC article.
-
Autophagy in toxicology: self-consumption in times of stress and plenty.J Appl Toxicol. 2012 Jul;32(7):465-79. doi: 10.1002/jat.1787. Epub 2012 Feb 15. J Appl Toxicol. 2012. PMID: 22334383 Free PMC article. Review.
References
-
- Watts C. The exogenous pathway for antigen presentation on major histocompatibility complex class II and CD1 molecules. Nat Immunol. 2004;5:685–92. - PubMed
-
- Cresswell P. Invariant chain structure and MHC class II function. Cell. 1996;84:505–7. - PubMed
-
- Sant AJ, Miller J. MHC class II antigen processing: biology of invariant chain. Curr Opin Immunol. 1994;6:57–63. - PubMed
-
- Riese RJ, Wolf PR, Bromme D, Natkin LR, Villadangos JA, Ploegh HL, Chapman HA. Essential role for cathepsin S in MHC class II-associated invariant chain processing and peptide loading. Immunity. 1996;4:357–66. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

