Stromal cells differentially regulate neutrophil and lymphocyte recruitment through the endothelium
- PMID: 20518822
- PMCID: PMC2992690
- DOI: 10.1111/j.1365-2567.2010.03307.x
Stromal cells differentially regulate neutrophil and lymphocyte recruitment through the endothelium
Abstract
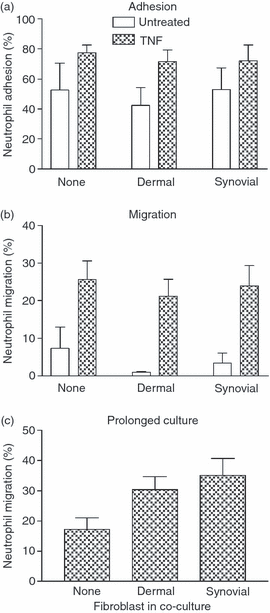
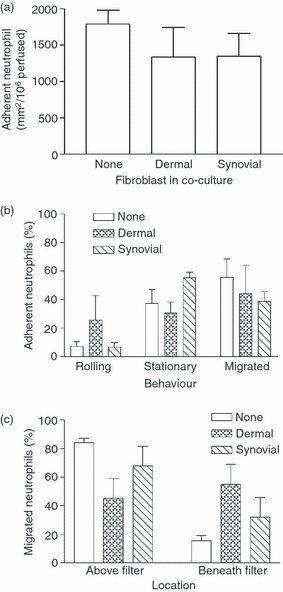
Stromal fibroblasts modify the initial recruitment of leucocytes by endothelial cells (EC), but their effects on subsequent transendothelial migration remain unclear. Here, EC and dermal or synovial fibroblasts were cultured on opposite surfaces of 3-μm pore filters and incorporated in static or flow-based migration assays. Fibroblasts had little effect on tumour necrosis factor-α-induced transendothelial migration of neutrophils, but tended to increase the efficiency of migration away from the endothelium. Surprisingly, similar close contact between EC and fibroblasts strongly reduced lymphocyte migration in static assays, and nearly abolished stable lymphocyte adhesion from flow. Fibroblasts did not alter endothelial surface expression of adhesion molecules or messenger RNA for chemokines. Inhibition of attachment did not occur when EC-fibroblast contact was restricted by using 0.4-μm pore filters, but under these conditions pre-treatment with heparinase partially inhibited adhesion. In the 3-μm pore co-cultures, inhibition of metalloproteinase activity partially recovered lymphocyte adhesion, but addition of CXCL12 (SDF-1α) to the endothelial surface did not. Hence, the ability of EC to present activating chemokines for lymphocytes may have been enzymatically inhibited by direct contact with fibroblasts. To avoid contact, we cultured EC and fibroblasts on separate 3-μm pore filters one above the other. Here, fibroblasts promoted the transendothelial migration of lymphocytes. Fibroblasts generate CXCL12, but blockade of CXCL12 receptor had no effect on lymphocyte migration. While stromal cells can provide signal(s) promoting leucocyte migration away from the sub-endothelial space, direct cell contact (which might occur in damaged tissue) may cause disruption of chemokine signalling, specifically inhibiting lymphocyte rather than neutrophil recruitment.
© 2010 The Authors. Immunology © 2010 Blackwell Publishing Ltd.
Figures





Similar articles
-
Fibroblasts from different sites may promote or inhibit recruitment of flowing lymphocytes by endothelial cells.Eur J Immunol. 2009 Jan;39(1):113-25. doi: 10.1002/eji.200838232. Eur J Immunol. 2009. PMID: 19130557 Free PMC article.
-
Adipokines and Inflammation Alter the Interaction Between Rheumatoid Arthritis Synovial Fibroblasts and Endothelial Cells.Front Immunol. 2020 Jun 2;11:925. doi: 10.3389/fimmu.2020.00925. eCollection 2020. Front Immunol. 2020. PMID: 32582145 Free PMC article.
-
Mesenchymal Stromal Cells as Active Regulators of Lymphocyte Recruitment to Blood Vascular Endothelial Cells.Methods Mol Biol. 2017;1591:121-142. doi: 10.1007/978-1-4939-6931-9_9. Methods Mol Biol. 2017. PMID: 28349479
-
Novel chemokine functions in lymphocyte migration through vascular endothelium under shear flow.J Leukoc Biol. 2001 Jun;69(6):860-6. J Leukoc Biol. 2001. PMID: 11404368 Review.
-
Modulation of endothelial responses by the stromal microenvironment: effects on leucocyte recruitment.Biochem Soc Trans. 2007 Nov;35(Pt 5):1161-2. doi: 10.1042/BST0351161. Biochem Soc Trans. 2007. PMID: 17956301 Review.
Cited by
-
Regulation of chemokine CCL5 synthesis in human peritoneal fibroblasts: a key role of IFN-γ.Mediators Inflamm. 2014;2014:590654. doi: 10.1155/2014/590654. Epub 2014 Jan 9. Mediators Inflamm. 2014. PMID: 24523572 Free PMC article.
-
Comparative Ability of Mesenchymal Stromal Cells from Different Tissues to Limit Neutrophil Recruitment to Inflamed Endothelium.PLoS One. 2016 May 12;11(5):e0155161. doi: 10.1371/journal.pone.0155161. eCollection 2016. PLoS One. 2016. PMID: 27171357 Free PMC article.
-
Adipogenic Differentiation of Mesenchymal Stem Cells Alters Their Immunomodulatory Properties in a Tissue-Specific Manner.Stem Cells. 2017 Jun;35(6):1636-1646. doi: 10.1002/stem.2622. Epub 2017 Apr 24. Stem Cells. 2017. PMID: 28376564 Free PMC article.
-
Endogenous Galectin-9 Suppresses Apoptosis in Human Rheumatoid Arthritis Synovial Fibroblasts.Sci Rep. 2018 Aug 27;8(1):12887. doi: 10.1038/s41598-018-31173-3. Sci Rep. 2018. PMID: 30150656 Free PMC article.
-
Importance of lymphocyte-stromal cell interactions in autoimmune and inflammatory rheumatic diseases.Nat Rev Rheumatol. 2021 Sep;17(9):550-564. doi: 10.1038/s41584-021-00665-4. Epub 2021 Aug 3. Nat Rev Rheumatol. 2021. PMID: 34345021 Review.
References
-
- Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–89. - PubMed
-
- Luu NT, Rainger GE, Nash GB. Differential ability of exogenous chemotactic agents to disrupt transendothelial migration of flowing neutrophils. J Immunol. 2000;164:5961–9. - PubMed
-
- Piali L, Weber C, LaRosa G, Mackay CR, Springer TA, Clark-Lewis I, Moser B. The chemokine receptor CXCR3 mediates rapid and shear-resistant adhesion-induction of effector T lymphocytes by the chemokines IP10 and Mig. Eur J Immunol. 1998;28:961–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

