Growth hormone releasing hormone induces the expression of nitric oxide synthase
- PMID: 20518847
- PMCID: PMC3822627
- DOI: 10.1111/j.1582-4934.2010.01096.x
Growth hormone releasing hormone induces the expression of nitric oxide synthase
Abstract
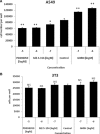
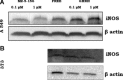
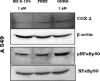
Growth hormone releasing hormone (GHRH) and its receptors are expressed in a wide variety of human tumours and established cancer cell lines and are involved in carcinogenesis. In addition, GHRH antagonists exert an antitumour activity in experimental cancer models. Recent studies indicate that the mechanisms involved in the mediation of the effects of GHRH include the regulation of the metabolism of the reactive oxygen species. This work demonstrates the expression of GHRH receptors and GHRH in the A549 human lung cancer cell line and shows that the mitogenic effect of GHRH in these cells is dependent on the activation of the extracellular receptor kinase (ERK)1/2 pathway. The action of GHRH can be suppressed by GHRH antagonist MZ-5-156 and mitogen activated protein kinase (MAPK) inhibitor PD 098059. These results are reflected in the effect in the proliferating cell nuclear antigen. In addition, our study shows that GHRH increases the expression of the inducible nitric oxide synthase, an enzyme which is strongly involved in various human diseases, including cancer and augments key intracellular regulators of its expression, such as pNF (nuclear factor)κBp50 and cyclooxygenase 2. GHRH antagonist MZ-5-156 counteracts the effects of GHRH in these studies, indicating that this class of peptide antagonists may be useful for the treatment of diseases related to increased oxidative and nitrosative stress.
© 2011 The Authors Journal of Cellular and Molecular Medicine © 2011 Foundation for Cellular and Molecular Medicine/Blackwell Publishing Ltd.
Figures




Similar articles
-
Antagonists of growth hormone-releasing hormone inhibit the proliferation of human benign prostatic hyperplasia cells.Prostate. 2010 Jul 1;70(10):1087-93. doi: 10.1002/pros.21142. Prostate. 2010. PMID: 20232355
-
Antagonists of growth hormone-releasing hormone (GHRH) reduce prostate size in experimental benign prostatic hyperplasia.Proc Natl Acad Sci U S A. 2011 Mar 1;108(9):3755-60. doi: 10.1073/pnas.1018086108. Epub 2011 Feb 14. Proc Natl Acad Sci U S A. 2011. PMID: 21321192 Free PMC article.
-
GHRH antagonist MZ-5-156 increases the expression of AMPK in A549 lung cancer cells.Cell Cycle. 2011 Nov 1;10(21):3714-8. doi: 10.4161/cc.10.21.17904. Epub 2011 Nov 1. Cell Cycle. 2011. PMID: 22041656
-
Growth Hormone-Releasing Hormone in Lung Physiology and Pulmonary Disease.Cells. 2020 Oct 21;9(10):2331. doi: 10.3390/cells9102331. Cells. 2020. PMID: 33096674 Free PMC article. Review.
-
Antineoplastic action of growth hormone-releasing hormone (GHRH) antagonists.Recent Pat Anticancer Drug Discov. 2012 Jan;7(1):56-63. doi: 10.2174/157489212798358010. Recent Pat Anticancer Drug Discov. 2012. PMID: 21854358 Review.
Cited by
-
P53, GHRH, inflammation and cancer.EBioMedicine. 2018 Nov;37:557-562. doi: 10.1016/j.ebiom.2018.10.034. Epub 2018 Oct 19. EBioMedicine. 2018. PMID: 30344124 Free PMC article. Review.
-
Growth hormone-releasing hormone antagonists protect against hydrochloric acid-induced endothelial injury in vitro.Environ Toxicol Pharmacol. 2023 Apr;99:104113. doi: 10.1016/j.etap.2023.104113. Epub 2023 Mar 20. Environ Toxicol Pharmacol. 2023. PMID: 36940786 Free PMC article.
-
P53 supports endothelial barrier function via APE1/Ref1 suppression.Immunobiology. 2019 Jul;224(4):532-538. doi: 10.1016/j.imbio.2019.04.008. Epub 2019 Apr 18. Immunobiology. 2019. PMID: 31023490 Free PMC article.
-
Involvement of the unfolded protein response in the protective effects of growth hormone releasing hormone antagonists in the lungs.J Cell Commun Signal. 2021 Mar;15(1):125-129. doi: 10.1007/s12079-020-00593-0. Epub 2020 Nov 13. J Cell Commun Signal. 2021. PMID: 33185812 Free PMC article.
-
Activity of the growth hormone-releasing hormone antagonist MIA602 and its underlying mechanisms of action in sarcoidosis-like granuloma.Clin Transl Immunology. 2021 Jul 5;10(7):e1310. doi: 10.1002/cti2.1310. eCollection 2021. Clin Transl Immunology. 2021. PMID: 34257968 Free PMC article.
References
-
- Schally AV, Varga JL, Engel JB. Antagonists of growth-hormone-releasing hormone: an emerging new therapy for cancer. Nat Clin Pract Endocrinol Metab. 2008;4:33–43. - PubMed
-
- Schally AV. New approaches to the therapy of various tumors based on peptide analogues. Horm Metab Res. 2008;40:315–22. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

