GLP-1 inhibits and adrenaline stimulates glucagon release by differential modulation of N- and L-type Ca2+ channel-dependent exocytosis
- PMID: 20519125
- PMCID: PMC4310935
- DOI: 10.1016/j.cmet.2010.04.007
GLP-1 inhibits and adrenaline stimulates glucagon release by differential modulation of N- and L-type Ca2+ channel-dependent exocytosis
Abstract
Glucagon secretion is inhibited by glucagon-like peptide-1 (GLP-1) and stimulated by adrenaline. These opposing effects on glucagon secretion are mimicked by low (1-10 nM) and high (10 muM) concentrations of forskolin, respectively. The expression of GLP-1 receptors in alpha cells is <0.2% of that in beta cells. The GLP-1-induced suppression of glucagon secretion is PKA dependent, is glucose independent, and does not involve paracrine effects mediated by insulin or somatostatin. GLP-1 is without much effect on alpha cell electrical activity but selectively inhibits N-type Ca(2+) channels and exocytosis. Adrenaline stimulates alpha cell electrical activity, increases [Ca(2+)](i), enhances L-type Ca(2+) channel activity, and accelerates exocytosis. The stimulatory effect is partially PKA independent and reduced in Epac2-deficient islets. We propose that GLP-1 inhibits glucagon secretion by PKA-dependent inhibition of the N-type Ca(2+) channels via a small increase in intracellular cAMP ([cAMP](i)). Adrenaline stimulates L-type Ca(2+) channel-dependent exocytosis by activation of the low-affinity cAMP sensor Epac2 via a large increase in [cAMP](i).
Copyright 2010 Elsevier Inc. All rights reserved.
Figures

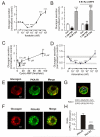
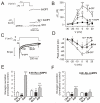
 ). Data are mean values ± S.E.M. of 7-13 experiments. *p<0.05, **p<0.01 and ***p<0.001 for comparisons between 8CPT-2Me-cAMP alone or 8CPT-2Me-cAMP in the simultaneous presence of isradipine vs. control. ††p<0.01 for values in simultaneous presence of 8CPT-2Me-cAMP and isradipine vs. 8CPT-2Me-cAMP alone. (C) Whole-cell Ca2+-currents recorded under control conditions (Ctrl), after intracellular application of 100 μM 8-CPT-2Me-cAMP (8-CPT) and in the presence of 8-CPT and 2 μM isradipine. (D) Peak Ca2+-currents recorded under control conditions (■), after intracellular addition of 8-CPT (○) and after intracellular application of 8-CPT when L-type Ca2+-channels were blocked by isradipine (2 μM). *p<0.05 and **p<0.01 for the stimulatory effects of 8-CPT (vs. Ctrl) and †p<0.05 and ††p<0.01 for the effect of isradipine (vs. 8-CPT). (n=5-10 experiments in each group) (E) Glucagon secretion from wildtype mouse islets under control conditions (Ctrl; 1 mM glucose), in the presence of 100 nM GLP-1 or 5 μM adrenaline (Adr) in the absence and presence of 10 μM 8-Br-Rp-cAMPS. n=6-8. **p<0.01 and ***p<0.001 vs. Ctrl in the absence or presence of 8-Br-Rp-cAMPS; ††p<0.01 and †††p<0.001 vs. corresponding value in the absence of 8-Br-Rp-cAMPS. Glucose was present at 1 mM. (F) As in C using islets from Epac2 null mice. n=5-8. **p<0.01 and ***p<0.001 vs. Ctrl in the absence or presence of 8-Br-Rp-cAMPS. ††p<0.05 vs. corresponding value in the absence of 8-Br-Rp-cAMPS.
). Data are mean values ± S.E.M. of 7-13 experiments. *p<0.05, **p<0.01 and ***p<0.001 for comparisons between 8CPT-2Me-cAMP alone or 8CPT-2Me-cAMP in the simultaneous presence of isradipine vs. control. ††p<0.01 for values in simultaneous presence of 8CPT-2Me-cAMP and isradipine vs. 8CPT-2Me-cAMP alone. (C) Whole-cell Ca2+-currents recorded under control conditions (Ctrl), after intracellular application of 100 μM 8-CPT-2Me-cAMP (8-CPT) and in the presence of 8-CPT and 2 μM isradipine. (D) Peak Ca2+-currents recorded under control conditions (■), after intracellular addition of 8-CPT (○) and after intracellular application of 8-CPT when L-type Ca2+-channels were blocked by isradipine (2 μM). *p<0.05 and **p<0.01 for the stimulatory effects of 8-CPT (vs. Ctrl) and †p<0.05 and ††p<0.01 for the effect of isradipine (vs. 8-CPT). (n=5-10 experiments in each group) (E) Glucagon secretion from wildtype mouse islets under control conditions (Ctrl; 1 mM glucose), in the presence of 100 nM GLP-1 or 5 μM adrenaline (Adr) in the absence and presence of 10 μM 8-Br-Rp-cAMPS. n=6-8. **p<0.01 and ***p<0.001 vs. Ctrl in the absence or presence of 8-Br-Rp-cAMPS; ††p<0.01 and †††p<0.001 vs. corresponding value in the absence of 8-Br-Rp-cAMPS. Glucose was present at 1 mM. (F) As in C using islets from Epac2 null mice. n=5-8. **p<0.01 and ***p<0.001 vs. Ctrl in the absence or presence of 8-Br-Rp-cAMPS. ††p<0.05 vs. corresponding value in the absence of 8-Br-Rp-cAMPS.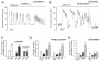

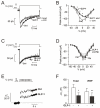
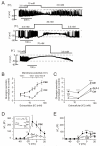
 ; n=6). *p<0.05 for effects of GLP-1 vs. control. (C-D) As in A-B but recorded under control conditions (Ctrl, black), 6 min after the addition of 10 μM 8-Br-Rp-cAMPS (Rp; dark grey) and 4 min after addition of 100 nM GLP-1 in the continued presence of 8-Br-Rp-cAMPS (Rp and GLP-1; light gray) in α-cells (E) Changes in membrane capacitance (ΔCm) elicited by ten voltage-clamp depolarizations from −70 mV to 0 mV under control conditions (1 mM glucose; □), after the addition of 10 μM 8-Br-Rp-cAMPS
; n=6). *p<0.05 for effects of GLP-1 vs. control. (C-D) As in A-B but recorded under control conditions (Ctrl, black), 6 min after the addition of 10 μM 8-Br-Rp-cAMPS (Rp; dark grey) and 4 min after addition of 100 nM GLP-1 in the continued presence of 8-Br-Rp-cAMPS (Rp and GLP-1; light gray) in α-cells (E) Changes in membrane capacitance (ΔCm) elicited by ten voltage-clamp depolarizations from −70 mV to 0 mV under control conditions (1 mM glucose; □), after the addition of 10 μM 8-Br-Rp-cAMPS  and in the simultaneous presence of 10 nM GLP-1 8-Br-Rp-cAMPS (●). (F) Histogram of the mean increase in membrane capacitance elicited by the entire train (Total) and increase evoked by the two first depolarizations (RRP). n=6; *p<0.05, **p<0.01.
and in the simultaneous presence of 10 nM GLP-1 8-Br-Rp-cAMPS (●). (F) Histogram of the mean increase in membrane capacitance elicited by the entire train (Total) and increase evoked by the two first depolarizations (RRP). n=6; *p<0.05, **p<0.01.References
-
- Berts A, Ball A, Gylfe E, Hellman B. Suppression of Ca2+ oscillations in glucagon-producing alpha 2-cells by insulin/glucose and amino acids. Biochim Biophys Acta. 1996;1310:212–216. - PubMed
-
- Bucci M, Roviezzo F, Brancaleone V, Lin MI, Di Lorenzo A, Cicala C, Pinto A, Sessa WC, Farneti S, Fiorucci S, et al. Diabetic mouse angiopathy is linked to progressive sympathetic receptor deletion coupled to an enhanced caveolin-1 expression. Arterioscler Thromb Vasc Biol. 2004;24:721–726. - PubMed
-
- Bylund DB, Bond RA, Eikenburg DC, Hieble JP, Hills R, Minneman KP, Parra S. IUPHAR-DB (IUPHAR) 2009.
-
- Cryer PE. Hypoglycaemia: the limiting factor in the glycaemic management of Type I and Type II diabetes. Diabetologia. 2002;45:937–948. - PubMed
SUPPLEMENTAL REFERENCES
-
- Barg S, Galvanovskis J, Gopel SO, Rorsman P, Eliasson L. Tight coupling between electrical activity and exocytosis in mouse glucagon-secreting alpha-cells. Diabetes. 2000;49:1500–1510. - PubMed
-
- Braun M, Ramracheya R, Amisten S, Bengtsson M, Moritoh Y, Zhang Q, Johnson PR, Rorsman P. Somatostatin release, electrical activity, membrane currents and exocytosis in human pancreatic delta cells. Diabetologia. 2009 - PubMed
-
- Braun M, Ramracheya R, Bengtsson M, Zhang Q, Karanauskaite J, Partridge C, Johnson PR, Rorsman P. Voltage-gated ion channels in human pancreatic {beta}-cells Electrophysiological characterization and role in insulin secretion. Diabetes. 2008 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
