Enhancement of gene targeting in human cells by intranuclear permeation of the Saccharomyces cerevisiae Rad52 protein
- PMID: 20519199
- PMCID: PMC2919737
- DOI: 10.1093/nar/gkq486
Enhancement of gene targeting in human cells by intranuclear permeation of the Saccharomyces cerevisiae Rad52 protein
Abstract
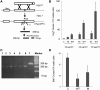
The introduction of exogenous DNA in human somatic cells results in a frequency of random integration at least 100-fold higher than gene targeting (GT), posing a seemingly insurmountable limitation for gene therapy applications. We previously reported that, in human cells, the stable over-expression of the Saccharomyces cerevisiae Rad52 gene (yRAD52), which plays the major role in yeast homologous recombination (HR), caused an up to 37-fold increase in the frequency of GT, indicating that yRAD52 interacts with the double-strand break repair pathway(s) of human cells favoring homologous integration. In the present study, we tested the effect of the yRad52 protein by delivering it directly to the human cells. To this purpose, we fused the yRAD52 cDNA to the arginine-rich domain of the TAT protein of HIV (tat11) that is known to permeate the cell membranes. We observed that a recombinant yRad52tat11 fusion protein produced in Escherichia coli, which maintains its ability to bind single-stranded DNA (ssDNA), enters the cells and the nuclei, where it is able to increase both intrachromosomal recombination and GT up to 63- and 50-fold, respectively. Moreover, the non-homologous plasmid DNA integration decreased by 4-fold. yRAD52tat11 proteins carrying point mutations in the ssDNA binding domain caused a lower or nil increase in recombination proficiency. Thus, the yRad52tat11 could be instrumental to increase GT in human cells and a 'protein delivery approach' offers a new tool for developing novel strategies for genome modification and gene therapy applications.
Figures


Similar articles
-
Roles of C-Terminal Region of Yeast and Human Rad52 in Rad51-Nucleoprotein Filament Formation and ssDNA Annealing.PLoS One. 2016 Jun 30;11(6):e0158436. doi: 10.1371/journal.pone.0158436. eCollection 2016. PLoS One. 2016. PMID: 27362509 Free PMC article.
-
Potentiation of gene targeting in human cells by expression of Saccharomyces cerevisiae Rad52.Nucleic Acids Res. 2005 Aug 16;33(14):4639-48. doi: 10.1093/nar/gki778. Print 2005. Nucleic Acids Res. 2005. PMID: 16106043 Free PMC article.
-
Rad52 Inverse Strand Exchange Drives RNA-Templated DNA Double-Strand Break Repair.Mol Cell. 2017 Jul 6;67(1):19-29.e3. doi: 10.1016/j.molcel.2017.05.019. Epub 2017 Jun 8. Mol Cell. 2017. PMID: 28602639 Free PMC article.
-
Nuclear localization of Rad52 is pre-requisite for its sumoylation.Biochem Biophys Res Commun. 2008 Jul 18;372(1):126-30. doi: 10.1016/j.bbrc.2008.05.020. Epub 2008 May 13. Biochem Biophys Res Commun. 2008. PMID: 18482582
-
Emerging non-canonical roles for the Rad51-Rad52 interaction in response to double-strand breaks in yeast.Curr Genet. 2020 Oct;66(5):917-926. doi: 10.1007/s00294-020-01081-z. Epub 2020 May 12. Curr Genet. 2020. PMID: 32399607 Free PMC article. Review.
Cited by
-
Inverted terminal repeats of adeno-associated virus decrease random integration of a gene targeting fragment in Saccharomyces cerevisiae.BMC Mol Biol. 2014 Feb 13;15:5. doi: 10.1186/1471-2199-15-5. BMC Mol Biol. 2014. PMID: 24521444 Free PMC article.
-
Recombination machinery engineering for precise genome editing in methylotrophic yeast Ogataea polymorpha.iScience. 2021 Feb 9;24(3):102168. doi: 10.1016/j.isci.2021.102168. eCollection 2021 Mar 19. iScience. 2021. PMID: 33665582 Free PMC article.
-
Enhancing Targeted Genomic DNA Editing in Chicken Cells Using the CRISPR/Cas9 System.PLoS One. 2017 Jan 9;12(1):e0169768. doi: 10.1371/journal.pone.0169768. eCollection 2017. PLoS One. 2017. PMID: 28068387 Free PMC article.
-
Methods for Enhancing Clustered Regularly Interspaced Short Palindromic Repeats/Cas9-Mediated Homology-Directed Repair Efficiency.Front Genet. 2019 Jun 17;10:551. doi: 10.3389/fgene.2019.00551. eCollection 2019. Front Genet. 2019. PMID: 31263478 Free PMC article. Review.
-
Genome editing in Kluyveromyces and Ogataea yeasts using a broad-host-range Cas9/gRNA co-expression plasmid.FEMS Yeast Res. 2018 May 1;18(3):foy012. doi: 10.1093/femsyr/foy012. FEMS Yeast Res. 2018. PMID: 29438517 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

