A review of nanoparticle functionality and toxicity on the central nervous system
- PMID: 20519209
- PMCID: PMC2943893
- DOI: 10.1098/rsif.2010.0158.focus
A review of nanoparticle functionality and toxicity on the central nervous system
Abstract
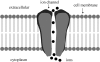
Although nanoparticles have tremendous potential for a host of applications, their adverse effects on living cells have raised serious concerns recently for their use in the healthcare and consumer sectors. As regards the central nervous system (CNS), research data on nanoparticle interaction with neurons has provided evidence of both negative and positive effects. Maximal application dosage of nanoparticles in materials to provide applications such as antibacterial and antiviral functions is approximately 0.1-1.0 wt%. This concentration can be converted into a liquid phase release rate (leaching rate) depending upon the host or base materials used. For example, nanoparticulate silver (Ag) or copper oxide (CuO)-filled epoxy resin demonstrates much reduced release of the metal ions (Ag(+) or Cu(2+)) into their surrounding environment unless they are mechanically removed or aggravated. Subsequent to leaching effects and entry into living systems, nanoparticles can also cross through many other barriers, such as skin and the blood-brain barrier (BBB), and may also reach bodily organs. In such cases, their concentration or dosage in body fluids is considered to be well below the maximum drug toxicity test limit (10(-5) g ml(-1)) as determined in artificial cerebrospinal solution. As this is a rapidly evolving area and the use of such materials will continue to mature, so will their exposure to members of society. Hence, neurologists have equal interests in nanoparticle effects (positive functionality and negative toxicity) on human neuronal cells within the CNS, where the current research in this field will be highlighted and reviewed.
Figures







References
-
- Cha K. E., Myung H. 2007. Cytotoxic effects of nanoparticles assessed in vitro and in vivo. J. Microbial Biotechnol. 17, 1573–1578. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
