In silico modelling of drug-polymer interactions for pharmaceutical formulations
- PMID: 20519214
- PMCID: PMC2943894
- DOI: 10.1098/rsif.2010.0190.focus
In silico modelling of drug-polymer interactions for pharmaceutical formulations
Abstract
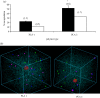
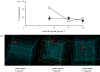
Selecting polymers for drug encapsulation in pharmaceutical formulations is usually made after extensive trial and error experiments. To speed up excipient choice procedures, we have explored coarse-grained computer simulations (dissipative particle dynamics (DPD) and coarse-grained molecular dynamics using the MARTINI force field) of polymer-drug interactions to study the encapsulation of prednisolone (log p = 1.6), paracetamol (log p = 0.3) and isoniazid (log p = -1.1) in poly(L-lactic acid) (PLA) controlled release microspheres, as well as the encapsulation of propofol (log p = 4.1) in bioavailability enhancing quaternary ammonium palmitoyl glycol chitosan (GCPQ) micelles. Simulations have been compared with experimental data. DPD simulations, in good correlation with experimental data, correctly revealed that hydrophobic drugs (prednisolone and paracetamol) could be encapsulated within PLA microspheres and predicted the experimentally observed paracetamol encapsulation levels (5-8% of the initial drug level) in 50 mg ml(-1) PLA microspheres, but only when initial paracetamol levels exceeded 5 mg ml(-1). However, the mesoscale technique was unable to model the hydrophilic drug (isoniazid) encapsulation (4-9% of the initial drug level) which was observed in experiments. Molecular dynamics simulations using the MARTINI force field indicated that the self-assembly of GCPQ is rapid, with propofol residing at the interface between micellar hydrophobic and hydrophilic groups, and that there is a heterogeneous distribution of propofol within the GCPQ micelle population. GCPQ-propofol experiments also revealed a population of relatively empty and drug-filled GCPQ particles.
Figures


Similar articles
-
Coarse-Grained Molecular Dynamics Simulations of Paclitaxel-Loaded Polymeric Micelles.Mol Pharm. 2022 Apr 4;19(4):1117-1134. doi: 10.1021/acs.molpharmaceut.1c00800. Epub 2022 Mar 4. Mol Pharm. 2022. PMID: 35243863
-
Co-micellization behavior of triblock copolymers in the presence of hydrophobic drug molecules: A simulation study.Colloids Surf B Biointerfaces. 2016 Dec 1;148:299-307. doi: 10.1016/j.colsurfb.2016.09.004. Epub 2016 Sep 5. Colloids Surf B Biointerfaces. 2016. PMID: 27619182
-
Physical characterisation and long-term stability studies on quaternary ammonium palmitoyl glycol chitosan (GCPQ)--a new drug delivery polymer.J Pharm Sci. 2014 Aug;103(8):2296-306. doi: 10.1002/jps.24026. Epub 2014 Jun 10. J Pharm Sci. 2014. PMID: 24916193
-
Principles of encapsulating hydrophobic drugs in PLA/PLGA microparticles.Int J Pharm. 2008 Dec 8;364(2):298-327. doi: 10.1016/j.ijpharm.2008.04.042. Epub 2008 May 7. Int J Pharm. 2008. PMID: 18621492 Review.
-
Microencapsulation of protein drugs for drug delivery: strategy, preparation, and applications.J Control Release. 2014 Nov 10;193:324-40. doi: 10.1016/j.jconrel.2014.09.003. Epub 2014 Sep 10. J Control Release. 2014. PMID: 25218676 Review.
Cited by
-
Manufacturing strategies to develop amorphous solid dispersions: An overview.J Drug Deliv Sci Technol. 2020 Feb;55:101459. doi: 10.1016/j.jddst.2019.101459. Epub 2019 Dec 11. J Drug Deliv Sci Technol. 2020. PMID: 32863891 Free PMC article.
-
Determining dominant driving forces affecting controlled protein release from polymeric nanoparticles.Biointerphases. 2017 May 19;12(2):02D412. doi: 10.1116/1.4983154. Biointerphases. 2017. PMID: 28525957 Free PMC article.
-
Common Internal Allosteric Network Links Anesthetic Binding Sites in a Pentameric Ligand-Gated Ion Channel.PLoS One. 2016 Jul 12;11(7):e0158795. doi: 10.1371/journal.pone.0158795. eCollection 2016. PLoS One. 2016. PMID: 27403526 Free PMC article.
-
Mechanistic Understanding From Molecular Dynamics Simulation in Pharmaceutical Research 1: Drug Delivery.Front Mol Biosci. 2020 Nov 25;7:604770. doi: 10.3389/fmolb.2020.604770. eCollection 2020. Front Mol Biosci. 2020. PMID: 33330633 Free PMC article. Review.
-
A hydrophobic gold surface triggers misfolding and aggregation of the amyloidogenic Josephin domain in monomeric form, while leaving the oligomers unaffected.PLoS One. 2013;8(3):e58794. doi: 10.1371/journal.pone.0058794. Epub 2013 Mar 19. PLoS One. 2013. PMID: 23527026 Free PMC article.
References
-
- Arshady R. 1990. Microspheres and microcapsules, a survey of manufacturing techniques. Part III: solvent evaporation. Polym. Eng. Sci. 30, 915–924. (10.1002/pen.760301506) - DOI
-
- Boussou C., van der Walle C. 2006. Poly(lactic acid-co-glycolic acid) microspheres. In Polymers in drug delivery (ed. Uchegbu I. F.), pp. 81–99. Boca Raton, FL: CRC Press.
-
- Daoulas K. C., Muller M. 2010. Comparison of simulations of lipid membranes with membranes of block copolymers. In Polymer membranes/biomembranes (eds Meier W. P., Knoll W.), pp. 197–233. Berlin, Germany: Springer-Verlag.
-
- Frenkel D., Smit B. 2002. Understanding molecular simulations: from algorithms to applications. San Diego, CA: Academic Press.
-
- Glotzer S. C., Paul W. 2002. Molecular and mesoscale simulation methods for polymer materials. Annu. Rev. Mater. Res. 32, 401–436. (10.1146/annurev.matsci.32.010802.112213) - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

